Story
The history of metallurgical production in our country goes back five thousand years. This is precisely the age of the first bronze objects found in the European part of Russia. Closer to the beginning of the 5th century AD, iron knives, axes, plows, sickles and other objects of labor and household use began to appear among the Slavic tribes.
The basis of metallurgy in the Moscow state until the 17th century was limonite, the so-called “swamp iron”. There were no explored reserves lying at a depth of several hundred meters underground at that time, and swamps were located everywhere. Just like the forest, which in those days played the role of metallurgical fuel.
The centers of iron production of that time were the following cities:
- Novgorod.
- Ustyug Iron.
- Rimov.
- Tula.
- Tikhvin.
- Olonets.
- Nut.
However, a significant amount of metal was purchased from Sweden and Germany. And the extraction and production of non-ferrous metals was practically absent until the 17th century (small copper sources in Pechora and the Olonets region did not satisfy the country’s needs).
The situation changed radically under Peter the Great, who decided to transfer exploration, mining and processing of ores into private hands. Although the first attempts to create domestic metallurgy by Ivan III and Ivan IV the Terrible were made earlier. In this regard, mention should be made of the Dutchman Vinius and a whole galaxy of famous Russian industrialists:
- Demidovs.
- Batashevs.
- Arekhovs.
- Krasilnikov.
- Mosolov.
Largely thanks to them, the Tula blast furnace and ironworks arose, and enterprises opened in the Urals. With the development of metallurgical production, Russia not only got rid of imports, but also became the largest producer and exporter of iron and cast iron.
As evidenced by the following indicators:
- 1725 1,165 thousand pounds of pig iron were smelted, which corresponds to more than 19 thousand tons.
- 1740 Russia produces 30 thousand tons more pig iron than the UK.
- In 1750, there were 72 iron factories and 29 copper smelters operating in the Russian Empire.
- 1767 83 thousand tons of cast iron.
- 1793 134 thousand tons of cast iron, 89,760 iron and steel.
- End of the 18th century. The country mines and processes: 2900 tons of copper, 1300 tons of lead, 28.7 tons of silver, 0.4 tons of gold.
In the early and mid-19th century, the growth rate of Russian metallurgy decreased significantly, which is explained by the establishment of state ownership of the largest enterprises, and as a consequence: the reluctance to update and improve equipment and establish new capitalist relations.
Metallurgy
Production of cast iron and steel on the territory of the Russian Empire in the 19th – early 20th centuries
| Years | Steel (thousand tons) | Cast iron (thousand tons) |
| 1830 | 178,72 | 108,8 |
| 1850 | 222,24 | 328,0 |
| 1870 | 350,40 | 221,20 |
| 1900 | 2865,60 | 2643,20 |
| 1913 | 4884,80 | 4803,20 |
Major political events at the beginning of the 20th century led to the liquidation of the mining industry and the shutdown of metallurgy enterprises. However, by the beginning of the Second World War, the USSR managed not only to restore, but also to significantly increase and modernize the industry. Domestic political and global economic problems of the late 20th and early 21st centuries significantly affected metal production. However, today the Russian Federation maintains high performance, remaining one of the world's largest exporters of this type of product.
Metallurgy of heavy metals
Copper production
The main stages of obtaining pure copper are the smelting of blister copper and its further refining. Blister copper is mined from ores, and the low concentration of copper in the Ural copper pyrites and its large volumes do not allow the transfer of production facilities from the Urals. The reserves are: cuprous sandstones, copper-molybdenum, copper-nickel ores.
Refining of copper and melting of secondary raw materials is carried out at enterprises that are remote from the sources of mining and primary smelting. They are favored by the low cost of electricity, since up to 5 kW of energy per hour is consumed to produce a ton of copper.
Metallurgical plant
The utilization of sulfur dioxide gases with subsequent processing served as the start for the production of sulfuric acid in the chemical industry. It produces phosphate mineral fertilizers from apatite residues.
Obtaining lead and zinc
The metallurgy of non-ferrous metals such as lead and zinc has complex territorial fragmentation. Ore is mined in the North Caucasus, Transbaikalia, Kuzbass and the Far East. And enrichment and metallurgical processing are carried out not only near ore extraction sites, but also in other territories with developed metallurgy.
Lead and zinc concentrates are rich in chemical elemental base. However, raw materials have different percentages of elements, which is why zinc and lead cannot always be obtained in pure form. Therefore, technological processes in the regions are different:
- In Transbaikalia, only concentrates are obtained.
- Lead and zinc concentrate are produced in the Far East.
- Zinc and lead concentrate are produced in Kuzbass.
- Redistribution is underway in the North Caucasus.
- Zinc is produced in the Urals.
Role in the economy
Metallurgy has always been the basis of the economy, since without its products the work of such industries as mechanical engineering, agriculture, construction, transport, and energy is impossible. The role of this industry is also great in the formation of the defense complex, the exploration of outer space and the creation of conditions for the development of the most advanced technologies. Ultimately, it is the current state of the metallurgical complex that reflects the level of the country’s scientific and technical potential and determines the development of all sectors of the national economy.
Only on its basis is it possible to implement new “breakthrough” technologies that can bring our country to the most advanced frontiers of the world economy. Because it is here that today the absolute majority of materials are created, endowed with special physical and chemical properties, without which the material realization of any achievements of progress is impossible.
so UNT / Geography / Lesson plans for geography 10th grade
Lesson 24. Non-ferrous metallurgy
24.08.2014 19353 0
Objectives: to form an idea of the location of non-ferrous metallurgy enterprises; determine factors for the location of non-ferrous metallurgy plants, identify changes in the location of enterprises in recent decades.
Equipment: map “Non-ferrous metallurgy of the world”, atlases.
During the classes
I. Survey
1. At the meeting in Cherepovets, a world map was demonstrated, on which a number of foreign cities were marked. They contain enterprises with which the leading enterprises of the city are planning to implement joint projects. Think about which cities are marked on this map? (Cities in which there are full-cycle ferrous metallurgy plants operating on imported coal and imported ore, for example, Baltimore, Toronto, etc.)
2. How can we explain the fact that from the creation of ferrous metallurgy plants they are now increasingly moving towards the creation of low-capacity plants?
3. Why in Germany the main centers of ferrous metallurgy were formed in the interior of the country, and in Italy - on the Mediterranean coast? Please provide at least two reasons. Name the centers. (Italy does not have its own raw materials and fuel. The country is forced to purchase raw materials. Therefore, enterprises are located on the coast. For example, Toronto.)
4. Which region of the world is the leader in iron ore mining? For steel production?
5. Which countries in the world export almost all of their iron ore?
6. Highlight the capitals of the two countries with the largest volumes of iron ore exports: Kinshasa, Georgetown, London, Santiago, Pretoria, Kingston, Monrovia, Conakry, Paramaribo.
7. What changes are taking place in the location of ferrous metallurgy enterprises at the present time?
8. Why are developing countries playing an increasingly important role in the smelting of ferrous metals? What stages of the metallurgical cycle are located here?
II. Testing (see appendix)
III. Learning new material
Stages of production of non-ferrous metals
Non-ferrous metallurgy is the oldest industry. It includes several stages: extraction of raw materials, enrichment, metal production, refining of some non-ferrous metals, production of alloys.
Low metal content in ore requires mandatory beneficiation. Since non-ferrous metal ores usually contain a large number of different metals, each component is separated sequentially during smelting.
Enrichment occurs in smelting furnaces, thus obtaining rough metal. But rough metal contains many impurities, so it is refined. Various profiles are obtained from the resulting pure metal.
Factors in the location of non-ferrous metallurgy enterprises The location of non-ferrous metallurgy enterprises occurs under the influence of various factors. Firstly, this is the raw material factor, since to obtain, for example, 1 ton of copper it is necessary to process 100 tons of ore, to obtain 1 ton of tin it is necessary to process 300 tons of raw materials. Secondly, the energy factor, the aluminum industry is a particularly energy-intensive industry. Therefore, aluminum industry plants will be located near power plants; in this case, hydroelectric power plants are especially attractive for the aluminum industry. Recently, the availability of secondary raw materials has been of great importance for the location of non-ferrous metallurgy plants. Remelting produces 1/5 of tin and up to 2/5 of copper. Basic metals in demand by the global economy Currently, non-ferrous metallurgy produces about 62 metals. But the main share falls on copper, aluminum, zinc, and lead. In the second half of the 20th century, the aluminum industry grew at a particularly rapid pace.
Ore mining areas and non-ferrous metals production areas Assignment: Based on the data from the atlas maps, analyze the ore mining areas and non-ferrous metals smelting areas, enter the analysis results in a table. Draw a conclusion about which countries focus on their own raw materials and which rely on imported ones.
III. Conversation following the work
After checking the quality of filling out the table, the teacher suggests comparing countries that mine non-ferrous metal ores and countries that produce pure metal.
1) What pattern can be identified in the location of plants for the production of crude and refined metals? (The stages of mining and beneficiation of ores are more represented in developing countries, this is due to the tectonic features of these territories; it is in developing countries that reserves of non-ferrous metal ores are concentrated. For example, the share of aluminum ores located in developing countries is 1/2, and the share of tin - 4/5 of the world's reserves. Production of pure metal is located mainly in developed countries, since this is a more environmentally friendly production, but also more energy-intensive.)
1) Which developing countries have the top floors of non-ferrous metallurgy production in their countries? (In recent decades, energy-intensive production of non-ferrous metallurgy can be afforded by not very developed countries, such as China, Brazil, Chile.)
2) In which branch of non-ferrous metallurgy the location of production has not changed? (In copper, raw materials are still the main factor in this industry.)
3) Why do China, Brazil, Chile, India, Venezuela locate and develop non-ferrous metallurgy? (This is due to several reasons, firstly, the presence of a raw material base in these countries, secondly, the presence of a fairly developed energy sector, and thirdly, dirty production from developed countries is transferred to these countries.)
4) Which branch of non-ferrous metallurgy is characterized by a gap between the areas of extraction and production of pure metal? {For aluminum.)
5) Why is the share of developing countries in the production of non-ferrous metals growing at an accelerated pace compared to developed countries? (They accept dirty industries from developed countries.)
6) Which country is the undisputed leader in all indicators of non-ferrous metallurgy? {USA.)
V. Homework
1. Having met at the international exhibition “Non-Ferrous Metals”, copper industry specialists from 6 countries started talking about how each of them spends their leisure time at home. Two turned out to be avid skiers, and one of them said that there is snow in the vicinity of the city most of the year and proudly reported that the plant where he works is the northernmost in the world. Two others complained about the constant heat, while one of them complained about the dry climate, and the second about tropical downpours and dampness. It turned out that five of them live near large lakes, however, not all of them swim in them - somewhere it’s too cold, and in two there is salt water (by the way, it turned out that two of them live in cities of the same name as the lakes ). Only one of them boasted that he swims in the ocean on weekends. Another replied that he needed a passport to go to the beach. Three are ski enthusiasts. Two are collectors of minerals, one of them called his peninsula a mineralogical museum, the other assured that his region had recently been a blank spot on the map, and minerals unknown to science could be found here. Representatives of which countries met at the exhibition? What are the names of the cities where they came from? Which of these countries produces the most ore? Which country smelts more metal?
2. Recall from the 9th grade course the importance of the mechanical engineering industry.
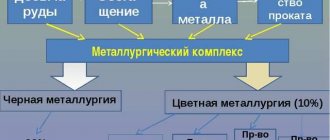
Ferrous metallurgy
One of the main industries providing heavy engineering and a number of other ministries:
- Steel.
- Cast iron.
- Ferroalloys.
- For rent.
Ferrous metallurgy provides up to 10% of the country's industrial production, with employment of 385.6 thousand people. At the same time, it is one of the largest components of the influx of foreign currency. Thus, in 2021, the export of ferrous metals brought $10.1 billion to the country’s budget.
Production Features
Ferrous metals are iron, manganese, chromium. Fossil ores of these metals are rich in the content of the desired component and can be mined in open pit and underground mines. The process of obtaining the final product itself includes a number of successive stages:
- Extraction and enrichment of iron, manganese and chrome ore, as well as non-metallic raw materials used in the production: refractory clays, fluxing limestones, molding materials.
- Pelleting is the formation of small volumes of raw materials with the properties necessary for blast furnace smelting.
- Smelting or manufacturing: carbon steel, cast iron.
- Production: rolled products, ferrous metal powders.
- Production of cast iron or steel pipes.
- Processing of scrap and waste ferrous metals for the purpose of their recycling.
Ferrous metallurgy
The technological cycle itself consists of the following productions:
- Cast iron and blast furnace.
- Electric steelmaking.
- Oxygen converter.
- Martenovskoe.
- Continuous casting.
- Rental.
Enterprises
The largest ferrous metallurgy enterprises:
- JSC United Metallurgical Company.
- OJSC "Pipe Metallurgical Company"
- OJSC "Holding"
- Mechel Group PJSC.
- PJSC Severstal.
- Public.
The main product of non-ferrous metallurgy. Characteristics of the industry
Metallurgy
The functional characteristics of non-ferrous metallurgy are determined by its following distinctive features:
- Non-ferrous metallurgy has the highest consumption of raw materials and materials among other industrial productions. To ensure its operation, significant volumes of raw materials are required. Basically, ore with a low content of valuable components (from 0.3–0.5 to 2.1%) is used for processing. The exception is the processing of bauxite to create aluminum.
- This industry has the most significant electricity and fuel consumption. The most energy-consuming industries are the lead, copper, nickel and cobalt industries.
- To ensure the uninterrupted operation of a non-ferrous metallurgy enterprise, a large number of labor resources are required, i.e. this industry, among other things, is labor-intensive.
Mining non-ferrous metallurgy is a labor-intensive process
- Enterprises in this production sector are mainly engaged in the processing of polymetallic ores.
- This industry consists of several mandatory stages. These include the stages of ore mining, its enrichment, metallurgical processing, and further processing of the resulting metal. Only the passage of all the listed stages constitutes a complete production process (cycle).
- Enterprises in the non-ferrous metallurgy industry are located geographically depending on the location of mineral resources. In this case, the natural resource factor is decisive.
- Non-ferrous metallurgy is considered one of the most dangerous in the industry for the environment. Its activities are associated with constant releases of large volumes of toxic substances.
Non-ferrous metallurgy
An industry engaged in the extraction and production of non-ferrous metals and their alloys, including: light, heavy, precious, rare, refractory. Compared to ferrous metallurgy, its production volumes are 20 times less, although its share in GDP is 7.8%, which is reflected in the number of personnel, amounting to 193 thousand people.
However, in terms of exports, non-ferrous metallurgy is significantly ahead of ferrous metallurgy. In 2021, the industry delivered to the international market:
- Non-ferrous metals worth $11.2 billion.
- Precious metals – by 11.2 billion dollars (gold – 124 tons).
- Rentals - by 2.4 billion dollars.
Production Features
Non-ferrous metals include all metals except iron and its alloys. Their production technology represents a certain sequence, which includes:
- Mining and beneficiation of ores.
- Smelting and sometimes also refining of metal.
- Receiving redistribution.
- Production of rolled metal.
Non-ferrous metallurgy
Distinctive features of production in non-ferrous metallurgy are:
- The raw material “poverty” of ores, compensated by a wide variety of accompanying elements.
- Increased demand for raw materials, water and energy resources.
- The complexity and multi-stage nature of a large number of different technological cycles used in the processing of raw materials and in the process of obtaining the final product.
It is quite logical that such specific features required the creation of multidisciplinary associations, sometimes combining chemistry, metallurgy and energy.
Enterprises
The leaders of non-ferrous metallurgy today are:
- JSC RUSAL Sayanogorsk Aluminum.
- JSC "Electrozinc"
- OJSC Sredneuralsk Copper Smelter.
- Evraz Vanadium Tula LLC.
- LLC "Mednogorsk Copper and Sulfur Combine".
- LLC "Novoangarsk Concentrating Plant"
- PJSC MMC Norilsk Nickel.
- PJSC RUSAL Bratsk Aluminum Plant.
- PJSC "Chelyabinsk Zinc Plant".
Basic products and intermediate products of metallurgical production.
⇐ PreviousPage 4 of 17Next ⇒Non-ferrous metallurgy is a complex branch of industrial production. The range of commercial products of non-ferrous metallurgy enterprises is quite wide and varied. This:
· non-ferrous metals and alloys in the form of ingots, cathodes, rolled products, etc.
· chemical products: sulfuric acid, elemental sulfur, copper and nickel sulfate, soda ash, potash, various chemical reagents (salts, oxides, hydroxides, etc.);
· mineral fertilizers: superphosphate, amophos, etc.;
· building materials: cement, mineral wool and asbestos slate products, crushed stone, granulated slag, slag paving stones, etc.;
· thermal and electrical energy;
· oxygen and argon.
In addition to commercial products, non-ferrous metallurgy enterprises produce numerous wastes and intermediate products of metallurgical production, the properties and qualities of which are very important for the technological processes of non-ferrous metallurgy. These include slags, mattes, dusts, gases, agglomerates and cakes, cakes, sludge, solutions, etc. Let us consider the general characteristics of some of the main products and the most important intermediate products of metallurgical technology obtained by processing most raw materials.
Metals
Metals are the main type of commercial products of metallurgical production. In non-ferrous metallurgy, depending on the technology used and the composition of the resulting metals, rough and refined metals are distinguished. Commodity products supplied to the consumer for further use for their intended purpose, as a rule, are refined metals.
Crude metals are metals contaminated with impurities.
Impurities include harmful and valuable elements - satellites of the base metal. Harmful impurities worsen the properties characteristic of a given metal (electrical conductivity, ductility, corrosion resistance, etc.) and make them unsuitable for direct use. Valuable satellites - noble metals, selenium, gallium, indium, rhenium and many others must be extracted along the way due to their fairly high price.
After purification (refining) of crude metals from impurities, refined metals are obtained. Depending on the quality of purification and the final content of impurities in the refined metal, the grade of the metal is determined. The purer the metal, the higher its cost (due to the high costs of cleaning it). In small quantities, some non-ferrous metallurgy enterprises produce metals of high (special) purity. The production of such metals is associated with large additional costs of labor, time and money, so their production is limited.
Steins
Matte is an alloy of sulfides of heavy non-ferrous metals (copper, nickel, lead, zinc, etc.) with iron sulfide, in which impurities are dissolved.
Mattes are an intermediate metal-containing product, the production of which is typical for the pyrometallurgy of copper, nickel and partially lead.
In the practice of non-ferrous metallurgy, copper, copper-nickel and polymetallic mattes are produced.
Approximate composition of factory mattes
| Matte | Cu | Ni | Pb | Zn | Fe | S |
| Copper | 10-60 | up to 0.5 | up to 1 | 1-6 | 30-50 | 23-26 |
| Copper-nickel | 5-10 | 5-13 | 40-60 | 24-27 | ||
| Nickel | 0.1-0.3 | 12-60 | 55-10 | 15-22 | ||
| Polymetal. | 10-30 | 10-20 | 5-10 | 20-40 | 13-22 |
They are formed in a liquid state and practically do not mix with liquid slags, which allows them to be separated from each other by settling. To successfully separate mattes and slags, it is necessary that the difference in their densities be at least 1 (g/cm3). The larger it is, the faster, other things being equal (viscosity of the slag, size of the matte droplets), the faster the settling.
The main components of copper matte are copper and iron sulfides - Cu2S and FeS. Ni3S2, Cu2S and FeS predominate in copper-nickel mattes. The characteristic features of copper and copper-nickel mattes are their approximately constant sulfur content and the presence of a certain amount of oxygen (up to 3-6% in poor mattes) in the form of iron oxides - FeO and Fe3O4.
Copper and copper-nickel mattes are good solvents (collectors) of all noble metals. In addition, depending on the composition of the feedstock, they may contain small amounts of selenium, tellurium, arsenic, antimony, bismuth, cadmium and other impurities.
Unlike copper and copper-nickel mattes, the sulfur content in nickel mattes varies more significantly. This is due to the fact that nickel mattes are usually metallized, i.e. in addition to sulfides, they also contain pure metals (Ni, Fe) in dissolved form. The higher the metallization of the matte, the lower its sulfur content. There is practically no oxygen in metallized nickel matte.
Metallurgical slags.
Slag is the second liquid (in rare cases solid) product of most metallurgical smelting.
Slag is an alloy of oxides formed mainly from gangue rock components and fluxes and products of oxidation reactions.
In addition to slag-forming components, real factory slags also contain a certain amount of extractable metals. When smelting for matte, a small amount of sulfides is also present in the slag.
With a relatively low content of valuable components in most ore smelting, slag is a waste product, i.e. waste from metallurgical production. However, in the future, with the development of metallurgical technology, they can again become a valuable raw material for the production of a number of non-ferrous metals, as well as iron and other valuable components.
The main components of non-ferrous metallurgy slag are SiO2, FeO and CaO. The total content of these three oxides usually ranges from 70 to 90 -95%. The CaO concentration rarely exceeds 6-8%. In most cases, therefore, non-ferrous metallurgy slags are basically iron silicate (FeO-SiO2).
In a number of non-ferrous metallurgy processes, depending on the composition of the processed raw materials and the technology used, metallurgical slags may contain Al2O3, MgO, Fe3O4, ZnO and some other oxides.
The most important physical and chemical properties of slag melts that affect smelting performance include fusibility, viscosity, density, surface properties and solubility of a metal-containing product in slag (the main properties and physical and chemical properties of metallurgical melts will be discussed later in this course). The physical and chemical properties of slag have a significant impact on the performance of the metallurgical process. In turn, the properties of the slag are significantly influenced by its composition and temperature.
The strong influence of slag composition and temperature on its properties leads to the need in each metallurgical process to select the optimal slag composition, which must satisfy certain technological and economic requirements. Melting the feedstock does not always ensure the production of optimal slags. In most cases, the composition of the slag has to be adjusted by introducing appropriate fluxes—mineral additives (quartz materials, limestone, etc.) into the initial charge.
⇐ Previous4Next ⇒
What makes your dreams come true? One hundred percent, unshakable confidence in your...
What Causes Trends in Stock and Commodity Markets Freight Train Theory Explained My first 17 years of market research consisted of trying to figure out when...
What does the IS operation and maintenance department do? Responsible for the safety of data (copying schedules, copying, etc.)…
System of Protected Areas in the USA The study of specially protected natural areas (SPNA) in the USA is of particular interest for many reasons...
Didn't find what you were looking for? Use Google search on the site:
Basic principles of placement
Due to their specific nature, metallurgical enterprises should be located in close proximity to:
- Places of occurrence and extraction of fossil ores.
- Sources and production of fuel: coal, coke.
- Water resources.
- Electric stations.
- Areas of consumption of finished products.
A developed transport infrastructure plays an important role in this. Production must be provided to one degree or another with: railway, road transport, water and fuel supply pipelines, as well as power lines.
Production of non-ferrous metals
Ministry of General and Vocational Technical Education
Moscow State Technical University “MAMI”
Department of Technology of Structural Materials
Production of non-ferrous metals.
Student: Zinoviev M.Yu.
Group: 5-AiU-1
1. Copper production.
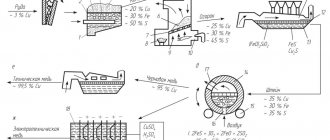
Copper is obtained mainly by the pyrometallurgical method, the essence of which is the production of copper from copper ores, including its beneficiation, roasting, smelting into an intermediate product - matte, smelting blister copper from matte and its purification from impurities (refining).
For the production of copper, copper ores containing 1–6% Cu, as well as waste copper and its alloys, are used. In ores, copper is usually found in the form of sulfur compounds, oxides or bicarbonates. Before smelting, copper ores are enriched and a concentrate is obtained. To reduce the sulfur content in the concentrate, it is subjected to oxidative roasting at a temperature. The resulting concentrate is melted in reverberatory or electric furnaces. At temperature, copper oxide (CuO) and higher iron oxides are reduced. The resulting copper oxide, reacting with, gives copper and iron sulfides fused and form matte, and molten iron silicates dissolve other oxides and form slag. After this, the molten copper matte is poured into converters and blown with air to oxidize copper and iron sulfides and produce blister copper. Blister copper contains 98.4-99.4% Cu and a small amount of impurities. This copper is poured into molds.
Blister copper is refined to remove harmful impurities and gases. First, fire refining is carried out in reverberatory furnaces. Impurities S, Fe, Ni, As, Sb and others are oxidized by atmospheric oxygen supplied through steel tubes immersed in molten blister copper. Then the gases are removed by removing the slag and immersing raw wood in copper. Water vapor mixes the copper and helps remove other gases. In this case, the copper oxidizes, and to free it from the bath of liquid copper, cover it with charcoal and immerse wooden poles in it. Dry distillation of wood immersed in copper produces hydrocarbons that reduce
After fire refining, copper of 99-99.5% purity is obtained. Pigs are cast from it for smelting copper alloys (bronze and brass) or plates for electrolytic refining.
Electrolytic refining is carried out to obtain copper free of impurities (99.5% Cu). Electrolysis is carried out in baths coated on the inside with vinyl plastic or lead. Anodes are made from fire-refined copper, and cathodes are made from sheets of pure copper. The electrolyte is an aqueous solution (10-16%) and (10-16%). When a direct current is passed, the anode dissolves, the copper goes into solution, and copper ions are discharged at the cathodes
Impurities (arsenic, antimony, bismuth, etc.) settle to the bottom of the bath, are removed and processed to extract these metals. The cathodes are unloaded, washed and melted in electric furnaces.
Aluminum production.
The essence of the aluminum production process is to obtain anhydrous, impurity-free aluminum oxide (alumina) followed by the production of metallic aluminum by electrolysis of dissolved alumina in cryolite.
The main raw materials for aluminum production are aluminum ores: bauxite, nepheline, alunite, kaolin. Bauxite is of greatest importance. They contain aluminum in the form of minerals - hydroxides, corundum and kaolinite. Aluminum is produced by electrolysis of alumina - aluminum oxide in molten cryolite with the addition of aluminum and sodium fluorides. Aluminum production includes the production of anhydrous, impurity-free aluminum (alumina); obtaining cryolite from fluorspar; Electrolysis of alumina in molten cryolite.
Alumina is obtained from bauxite by treating it with alkali: .
The resulting sodium aluminate is subjected to hydrolysis:
As a result, aluminum hydroxide crystals precipitate. Aluminum hydroxide is dehydrated in rotary kilns at temperature to produce dehydrated alumina.
To produce cryolite, hydrogen fluoride is first obtained from fluorspar, and then hydrofluoric acid. Hydrofluoric acid is introduced into the solution, resulting in the formation of seruluminic acid, which is neutralized with soda and cryolite is obtained, which precipitates: .
It is filtered and dried in drying drums.
Electrolysis of alumina is carried out in an electrolytic cell containing a bath of carbonaceous material. In the bath, a layer of 250-300 mm contains molten aluminum, which serves as a cathode, and liquid cryolite.
The anode device consists of a carbon anode immersed in an electrolyte. A direct current of 70-75 kA and a voltage of 4-4.5 V is supplied for electrolysis and heating of the electrolyte to a temperature of 1000C
The electrolyte consists of cryolite, alumina, AlF3 and NaF. Cryolite and
alumina in the electrolyte dissociates; Al3+ ion is discharged at the cathode and
aluminum is formed, and at the anode - O2- ion, which oxidizes carbon
anode to CO and CO2, which are removed from the bath through the ventilation system.
Aluminum collects at the bottom of the bath under a layer of electrolyte. It periodically
removed using a special device.
For normal operation of the bath, a little aluminum is left at its bottom.
Aluminum produced by electrolysis is called raw aluminum.
It contains metallic and non-metallic impurities and gases.
Impurities are removed by refining, for which chlorine is blown through
aluminum melt. The resulting vaporous aluminum chloride,
passing through the molten metal, it envelops particles of impurities,
which float to the surface of the metal, where they are removed. Chlorination
aluminum also helps remove Na, Ca, Mg and gases dissolved in
aluminum Then the liquid aluminum is kept in a ladle or electric furnace in
for 30-45 minutes at a temperature of 690-730° C for floating
non-metallic inclusions and gas release from metal. After
refining, the purity of primary aluminum is 99.5-99.85%.
3. MAGNESIUM PRODUCTION
For the production of magnesium, the most widely used method is the electrolytic method, the essence of which is to obtain pure anhydrous magnesium salts (magnesium chloride), electrolysis of these salts in a molten state and refining of metallic magnesium.
The main raw materials for the production of magnesium are carnallite (MgCl2*KCL*6H2O), magnesite (MgCO3), dolomite (CaCO3 • MgC03), bischofite (MgCl2*6H2O). The largest amount of magnesium is obtained from carnallite. First, the carnallite is enriched and dehydrated. Anhydrous carnallite (MgCl2• KS1) is used to prepare the electrolyte. Electrolysis is carried out in an electrolyzer lined with fireclay bricks. The anodes are graphite plates, and the cathodes are steel plates. The electrolyzer is filled with a molten electrolyte of the composition 10% MgCl2, 45% CaCI2, 30% NaCI, 15% KCl with small additions of NaF and CaF2. This composition of the electrolyte is necessary to lower its melting point (720 ± 10 ° C). To electrolytically decompose magnesium chloride, current is passed through the electrolyte. As a result, chlorine ions are formed, which move towards the anode. Magnesium ions move to the cathode and, after discharge, are released on the surface, forming droplets of liquid rough magnesium. Magnesium has a lower density than the electrolyte, so it floats to the surface, from where it is periodically removed with a vacuum ladle. Raw magnesium contains 5% impurities, so it is refined by remelting with fluxes. To do this, rough magnesium and flux consisting of MgCl;,, KS1, Bad,, CaF,, NaCI, CaCI;, are heated in an electric furnace to a temperature of 700-750″ C and mixed. In this case, non-metallic impurities turn into slag. Then the furnace is cooled to a temperature of 670°C and the magnesium is poured into molds into pigs.
4. TITANIUM PRODUCTION
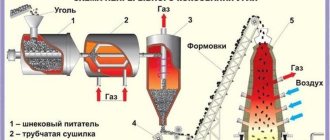
Titanium is produced by the magnesium-thermal method, the essence of which is the enrichment of titanium ores, smelting titanium slag from them, followed by the production of titanium tetrachloride from it and the reduction of the latter metallic titanium with magnesium.
The raw material for titanium production is titanomagnetite ores, from which ilmenite concentrate is isolated containing 40-45% TiO2, ~30% FeO, 20% Fe2O3 and 5-7% waste rock. This concentrate received its name from the presence of the ilmenite mineral Feo*TiO2.
Ilmenite concentrate is smelted in a mixture with charcoal and anthracite in ore-thermal furnaces, where iron and titanium oxides are reduced. The resulting iron is carburized, and cast iron is obtained, and lower titanium oxides turn into slag. Cast iron and slag are poured separately into molds. The main product of this process, titanium slag, contains 80–90% TiO2, 2–5% FeO and impurities of SiO2, Al2O3, CaO, etc. A by-product of this process, cast iron, is used in metallurgical production.
The resulting titanium slag is subjected to chlorination in special furnaces. At the bottom of the furnace there is a coal nozzle, which heats up when an electric current is passed through it. Titanium slag briquettes are fed into the furnace, and chlorine is fed through tuyeres into the furnace. At a temperature of 800-1250 ° C in the presence of carbon, titanium tetrachloride is formed, as well as chlorides CaCI2, MgCl2, etc. Ti02 + 2C + 2Cl2 = TiCl4 + 2CO.
Titanium tetrachloride is separated and purified from the remaining chlorides due to the difference in boiling point of these chlorides using the rectification method in special installations.
Titanium from titanium tetrachloride is reduced in reactors at a temperature of 950-1000 ° C. Pig magnesium is loaded into the reactor; After pumping out the air and filling the reactor cavity with argon, vaporous titanium tetrachloride is supplied inside it. A reaction occurs between liquid magnesium and titanium tetrachloride
2Mg+TiCl4=Ti+2MgCl2.
Solid titanium particles are sintered into a porous mass—a sponge—and liquid MgCl2 is released through the reactor tap hole.
Titanium sponge contains 35-40% magnesium and magnesium chloride.
To remove these impurities from a titanium sponge, it is heated to a temperature of 900-950 ° C in a vacuum.
Titanium sponge is melted using the vacuum-arc remelting method.
The vacuum in the furnace protects titanium from oxidation and helps clean it from impurities. The resulting titanium ingots have defects, so they are re-melted and used as consumable electrodes. After this, the purity of titanium is 99.6–99.7%. After secondary remelting, the ingots are used for pressure treatment.
Information about the work “Production of non-ferrous metals”
Section: Technology Number of characters with spaces: 10108 Number of tables: 0 Number of images: 0
Similar works
Technological basis for the production of non-ferrous metals: copper, aluminum, magnesium, titanium
40716
0
11
… . Therefore, it is considered normal for one or two anodic effects to occur per day in the electrolyzer. Many researchers have been studying the nature of the anode effect. Based on research conducted at the Moscow Institute of Non-Ferrous Metals and Gold under the guidance of Prof. A.I. Belyaev, we can conclude that the reason causing the anodic effect is the different wettability of the carbon anode...
Production of castings from non-ferrous metal alloys
70212
0
0
... insulate or warm up. The principle of directional solidification, realized and formulated during the development of the production of castings from aluminum and magnesium alloys, is now absolutely mandatory for obtaining high-quality castings from any alloys. The development of the scientific foundations for melting non-ferrous metal alloys, their crystallization, and mastering the technology for producing shaped castings and ingots is the merit of ...
Economic and geographical justification for the location of non-ferrous metallurgy enterprises
50833
5
0
... interregional differences in per capita production levels will continue until 2000, and especially sharply in the market development scenario. 4 Analysis of the activities of the largest non-ferrous metallurgy enterprises. 4.1 RAO Norilsk Nickel The year 1997 was a turning point for Norilsk Nickel. With the arrival of Oneximbank in the summer of 1996, there was hope for...
North Caucasus economic region. Ferrous and non-ferrous metallurgy.
111606
0
0
... - waste from roasting zinc concentrates. Sulfuric acid production includes the Electrozinc plant (Vladikavkaz) and other enterprises. An industry that complements the complex of the North Caucasus economic region is the coal industry, although due to the high cost of production, slow growth of labor productivity, and falling capital productivity, its efficiency is reduced. Main …
Industry competitiveness
The formation of the competitiveness of the metallurgical complex occurs in the conditions of established commodity-money relations in the international market and within the country. Accordingly, its performance is significantly influenced by the following factors:
- For the entire industry of the country as a whole, as a supplier of exported products:
- General economic and political situation in the world.
- The current situation in the global metallurgical market.
- International legislation together with the legal norms of product importing countries.
- For an individual manufacturer:
- Production and economic potential.
- Level of technical equipment.
- The location of the enterprise in relation to the supply of necessary resources and the organization of sales of finished products or products.
- Degree of integration.
The conducted studies show that the industry, one of the first in the country to become:
- Restructure production capacity.
- Eliminate ineffective equipment.
- Build industry structures, specialize, and then unite into complete completed cycles.
- Increase production of the most profitable and best-selling products.
- Engage in cost reduction.
- Solve environmental issues.
- Improve the social conditions of your employees.
Overall, it has achieved a high level of competitiveness.
Mctallurgy
Although some indicators: electricity consumption per unit of production (20-30% more than in Germany and the USA); labor costs (2.5 times higher than in Germany, the USA and Japan) remain at a very low level. Which requires further work to update production capacity and optimize the structure.
Main sub-sectors
The non-ferrous metallurgy includes several sub-sectors. The main one is the production of aluminum. It accounts for almost half of the world's smelting of non-ferrous metals. Bauxite is used as a raw material, from which alumina is obtained during processing. The main deposits are in China, Russia, Australia and Brazil.
Copper production accounts for approximately 25% of the market volume. For this purpose, enriched copper ore is used. Recycling of secondary raw materials is also important. The main deposits of copper ores are located in Central Africa, Chile, Russia, China, Canada and the USA.
Lead and zinc are produced from polymetallic ores. The largest volumes are mined in Mexico, the USA, Australia, China and Canada. These ores are processed in Australia, the USA, Japan, China and several EU countries.
The largest nickel deposits are located in Russia. It also acts as the world's main producer. The production of this metal accounts for 6% of the total world smelting volume. The raw material is nickel ore.
Tin is obtained by processing tin ores. The world's main deposits are located in Southeast Asia and Bolivia. Among the main smelting centers are Malaysia, China, Bolivia and Russia. The rest of the industry's products are manufactured on a much smaller scale. Most often these are local productions.
The production processes for various metals are similar, but depending on the specific natural resource they have certain differences, so the cycle can best be described using the example of aluminum production. It includes the following stages:
- Bauxite mining.
- Concentration by screening or washing. This is how the concentration of aluminum in the ore increases.
- Alumina production.
- Metal smelting.
- Production of aluminum ingots.
In Russia, this industry is one of the most developed. This is due to the presence of a large amount of raw materials, as well as a developed production base, which remained after the collapse of the USSR.
Development prospects
The main prospects for the development of Russian metallurgy are opening up by solving two main problems:
- Inconsistencies between the level of production and market requirements in terms of qualitative and quantitative indicators.
- Modernization, which must be carried out through the introduction of modern technologies of the highest scientific level.
- The most effective areas in this regard are:
- Further preferential use of blast-free smelting methods.
- Increased use of secondary resources. In light of the depletion of existing deposits and the difficulties of developing new ones, this direction is especially relevant!
- Increasing the share of alloys and other products of a high scientific and technological level.
- Introduction of energy-intensive but at the same time economical technologies.
- Resource and energy saving.
- Reducing environmental pollution.
All these areas are reflected in the development strategies of metallurgical production for 2014-2020 and for the future until 2030.
Author: Yuri Florinskikh All articles by this author
Latest articles by the author: The largest producers of milk and dairy products in the world Diamonds: properties, mining methods and applications
General characteristics and specifics of the industry
If we compare ferrous and non-ferrous metallurgy, the latter is more energy-intensive. It is characterized by complex production processes, since the ore content of the required element is usually low. It also often contains a large number of impurities. For example, copper ore contains a maximum of 5% copper. Pyrites are mined in the Urals. These are multicomponent ores, which contain about 30 chemical elements.
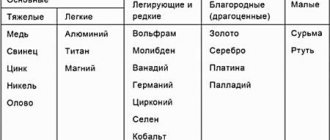
Non-ferrous metals are divided into several groups:
| Category | Peculiarities | Name of metals |
| Heavy | Dense and heavy | Copper, nickel, zinc, plumbum |
| Lungs | Low specific density and weight | Aluminum, lithium, titanium |
| Small | They are companions of heavy metals | Cobalt, antimony, mercury, cadmium |
| Alloying | Used for the production of steel and other alloys | Molybdenum, tungsten, vanadium |
| Noble | Used in microelectronics and for making jewelry | Gold, silver, platinum |
| Rare earth | May have different properties | Tantalum, niobium, yttrium, etc. |
Non-ferrous metal ores contain little extractable element, so to obtain a ton of copper it is necessary to process about 100 tons of rock. Most often, production complexes are located near the raw material base.
Also, processing ore requires a large amount of electricity. Energy costs account for about 50% of all production costs.
Non-ferrous metallurgy is divided into industries based on the type of element being mined. The largest production volumes come from the following metals:
- Copper.
- Aluminum.
- Cobalt and nickel.
- Tin.
- Lead and zinc.
- Gold.
Nickel production is associated with nickel ore mining sites. The main deposits are located in the Norilsk region of Siberia and on the Kola Peninsula. Most branches of metallurgy are characterized by multi-stage production stages. During waste disposal, other materials are obtained. They are then used in the mechanical engineering, construction and chemical industries.