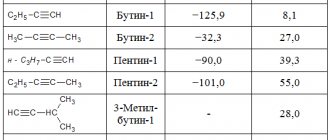
Gas welding is actively used in industry, which will be in demand in the national economy for a long time. The reason for this is that the burner flame temperature can reach 3150°C.
This temperature allows you to weld not only ordinary steel, but also alloyed and non-ferrous metals that a welding machine cannot weld with conventional electrodes. In addition, a gas torch can effectively cut metal of large thickness and various cross-section configurations. These flame characteristics are achieved by mixing acetylene and oxygen before combustion. Releases acetylene carbide gas for welding. It was invented by the German chemist Friedrich Wöhler in 1862. To create it, he placed a zinc-calcium alloy in hot coals.
What is carbide for welding
Carbide consists of two chemical elements: carbon and calcium, so its full name is calcium carbide. After production is completed, the finished product looks like pieces of hardened gray or brown cement. When it reacts with water, it heats up very much and releases a large volume of gas - acetylene, which has a pungent chemical odor. If you mix 560 ml of water with 1000 g of carbide, then as a result of their reaction, 372 liters of acetylene will be released and 1160 g of slaked lime will be formed.
This substance is obtained by fusing quicklime (calcium oxide) and coke in an electric furnace at a temperature of 1900°C - 2300°C. Modern enterprises for the production of calcium carbide use ore reduction furnaces with a power of 60 to 80 megavolts - amperes, with square or round baths.
Calcium carbide furnace
Welding using this substance consists of the following steps:
- A powdery mixture is created from coking coal and lime;
- then it is placed in an electric arc furnace and brought to melting;
- the alloy is poured into molds and cooled;
- the resulting carbide bars are removed and placed in a crushing chamber to give the required fraction.
The resulting material has a density of 2.2 g/cm3. Its composition includes about 77% CaC2 and 23% lime with various impurities. The larger the piece of carbide, the longer it takes to decompose during the reaction, but a larger volume of acetylene is released. However, if fine-grained carbide is used during welding, the reaction can be so violent that it can lead to an explosion.
This is what calcium carbide looks like
Calcium carbide as a “fuel” for welding.
As mentioned above, when welding, carbide reacts actively with water, releasing a huge amount of heat and acetylene gas. This feature complicates the storage of carbide, so to preserve it, the substance is placed in sealed roofing steel tanks with a capacity of 100 and 130 kilograms. Since carbide releases highly flammable acetylene, it is vital to avoid sparks or open flames when opening these cans.
Calcium carbide dust - particles up to 2 millimeters - is not suitable for use, since it dissolves almost immediately in water and at the same time, the likelihood increases that the use of such a composition will lead to an explosion of the entire cylinder.
For the curious, one kilogram of calcium carbide, depending on the purity and size of the pieces, upon contact with water can release more than 250 dm3 of acetylene!
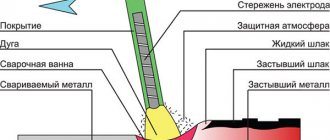
Calcium carbide is actively used during gas welding and cutting. When burned with oxygen, acetylene can reach a highest melting point of up to 3150 degrees Celsius, which makes it indispensable when working with refractory metals, because welding or cutting requires a temperature twice the melting degree of the metal itself.
For safe use, acetylene is produced in special generators (Fig. 2) based on calcium carbide or natural gas, oil and coal.
The second method of producing acetylene from natural gas, oil and coal is cheaper than using calcium carbide, by about 30 - 40%.
Safety precautions
Not only acetylene gas itself, but also dry carbide are explosive substances. Therefore, when welding, it is necessary to strictly adhere to the safety measures prescribed by the instructions. To avoid emergencies that could lead to tragedy, you must adhere to the following rules:
- within a radius of 10 m from the acetylene generator and barrels with the substance there should be no sources of fire or sparks, including the operating burner itself;
- do not use carbide for welding in a dusty state and with a fraction of about 2 mm, since a violent reaction will cause an explosion;
- Carbide should be stored in a sealed container away from sources of fire, moisture, water and sewer pipes;
- do not allow the substance to come into contact with mucous membranes, eyes or skin, so use a respirator, gloves and goggles when working with it;
- if the material does get on the body, thoroughly rinse the affected areas with plenty of water;
- if welding is carried out indoors, it is necessary to ensure good ventilation and also remove all flammable materials;
- when operating in basements, the generator must be installed outdoors; it is prohibited to place it in any basements, as well as in residential buildings;
- Before charging the generator, you must ensure the integrity of its housing and equipment;
- during the welding process, it is necessary that the pressure gauge is clearly visible to the welder and his assistant, and that the apparatus always stands level;
- It is forbidden to open a generator under pressure;
- upon completion of welding work, it is necessary to carefully select undecomposed carbide, slaked lime, and thoroughly rinse the apparatus with water;
- reuse of non-decomposed material is prohibited;
- acetylene cylinders are transported and stored with fuses screwed onto the valves;
- it is necessary to buy carbide only from licensed manufacturers and sellers, whose authority has been earned through years of conscientious cooperation with clients;
- Welding using homemade generators is prohibited, as this can lead to tragic events.
The process of producing acetylene from carbide
A device for producing acetylene from calcium carbide is called an acetylene generator. The equipment can be mobile or stationary. Mobile generators are used mainly during repair work, while stationary ones are used at sites with large volumes of welding processes. How is acetylene obtained from calcium carbide? The operating principle of the generator is as follows:
- The chamber intended for gas formation is filled with water in the calculated volume.
- The required amount of calcium carbide is loaded into the gas-forming chamber through a special hopper. It is prohibited to use carbide dust, as it can lead to instant gas release and depressurization of the device.
- Carbide for welding is supplied from the hopper to the chamber in automatic portions.
- As each portion is fed, the pressure inside the chamber increases. Its reduction serves as a command to load the subsequent part of the carbide.
- During the interaction of calcium carbide with water, acetylene is released, which is fed through a selector into the hose leading to the welding torch.
The secondary product in the form of slaked lime is removed from the generator using a special hopper. When working with an acetylene generator, you must remember that it is strictly forbidden to smoke or use electric tools in the immediate vicinity of it. The gas burner should be no closer than 10 meters. This is the size that should be the minimum length of the welding hose.
If there is a need for welding work and you need to decide where to get carbide, then the safest thing to do is contact direct suppliers or buy from online stores that organize delivery by a transport company.
- Exterior finishing
Application area
Calcium carbide (Calcium carbide) is used to produce calcium cyanamide (by reaction with nitrogen), from which cyanide compounds and fertilizers are synthesized, for the production of carbide-urea plant growth regulators and carbide powder reagent.
Autogenous work and lighting, the production of acetylene black and other materials: synthetic rubber, alkonitrile, styrene, vinyl chloride, acetic acid, acetylene chloride derivatives, artificial resins, ethylene, acetone, etc., cannot be done without this substance. It is also used in the gas welding process, production of carbide lamps.
From a special fraction of calcium carbide (processed using waste and substandard raw materials) by reaction with water, acetylene gas and a by-product - slaked lime - are obtained. This procedure is accompanied by the release of a significant amount of heat. The volume of gas produced depends on the purity of calcium carbide (the purer the material, the more acetylene will be released) and varies between 235-285 liters from 1 kg of carbide.
Theoretically, 0.56 liters of water are required to decompose 1 kg of calcium carbide, but in practice, 5 to 26 liters of liquid are used to better cool the acetylene and ensure the safety of the process. The rate of decomposition will depend on the granulation and purity of the starting material, as well as on the temperature and purity of the water (the purer and smaller the size, the higher the temperature, the faster the reaction rate).
Applications of boron carbide
Boron carbide formula B4C. It is also called boron carbide. It appears as grey-black shiny crystals with a rhombic lattice pattern.
And although it was discovered back in the 19th century, it is in great demand for powder metallurgy today. In particular, the high-pressure sintering method makes it possible to create durable products without pores. Diffusion processes in combination with B4C proceed very slowly and interfere with the compaction of free sintering. That is why this method is used. Very resistant to various aggressive environments. Chemically stable and has low specific gravity.
Let's take a closer look at the properties and applications of boron carbide.
Properties
It is worth noting that boron carbide has a wide range of chemical, mechanical and physical properties.
Belongs to the class of the most chemically resistant. Oxidation occurs in open air at temperatures above 600°C. Hardness comparable to diamonds. Very durable. The density of boron carbide is 2.52 g/cm3. The key to the application is the fact that boron carbide does not dissolve in either water or highly concentrated acids. Decomposes when the substance is placed in alkaline solutions at boiling.
If we are talking about nitrogen, phosphorus, sulfur, then it reacts when it reaches a temperature value of more than 1250 degrees Celsius). Formation of boron trichloride and carbon when reacting with chlorine at temperatures above 1000 degrees Celsius. Melts at temperatures above 2300 degrees.
Storage and transportation
Boron carbide should be stored only in its original packaging in indoor warehouses where it is as dry as possible and has a good ventilation system. By allowing violations in the conditions, it is impossible to exclude that a number of physical and chemical properties will be violated. This is unacceptable.
Transportation of these products can be carried out by any means. By contacting our company, buyers can independently determine how it will be transported and by which transport organization.
Application
It occupies one of the first places among other materials that can be used for current industrial processes. Due to its quality characteristics, boron carbide is widely used in modern economic processes. In particular, it is important for sandblasting surfaces - wooden furniture, glass, metal, building facades and fences.
The scope of application of boron carbide depends on the size of the crystals, so it can be used:
- as a fireproof, grinding and micro-grinding material (using a sandblasting machine produces excellent smooth surfaces);
- for abrasive spraying (for tools, discs, wheels, attachments);
- ultrasonic treatment of natural stones and products made from them (boron carbide powder makes them more beautiful and aesthetic; further processing of the stone is not necessary);
- jewelry making (due to its strong hardness for separating precious and semi-precious stones and cutting them);
- performing lapping operations (one-dimensional grinding powders guarantee maximum lapping cleanliness, even with minor impact on precise conical and shaped surfaces);
- for grinding cutters made of hard alloys (the speed of the grinding process is quite high, while the powder grains are reduced);
- diffuse boriding of refractory metals and steel;
- production of ceramic material;
- in nuclear energy as a material for rods that regulate the course of nuclear reactions;
- as a protective layer for laboratory vessels and containers.
Experts have found that if you mix the compound and diamond dust, its abrasive characteristics will become higher. The level of resistance to oxidative reactions will also increase. Used in large production and at home.
In our company you can submit a remote purchase application. To do this, you will need to indicate the required name and quantity in an email. We guarantee prompt feedback.
How is carbide obtained?
First about calcium carbide. Its production is in demand. And although such factories require large expenses, especially when it comes to electricity, enterprises do not abandon the usual manufacturing method. Because the demand for such products is in no hurry to fall. After all, it is hardly possible to imagine at least a single construction site without acetylene. To save on electricity, such enterprises are opened in countries with a large number of hydroelectric power stations, in Canada, for example.
Why not switch to working with methane, since such a volatile gas can also be obtained from it? Yes, because calcium carbide produces an almost pure product, it is not difficult to bring it to 98 percent gas. And it is much easier to transport than the one obtained with the participation of methane.
The main object in such industries are electric furnaces. They are loaded with hard coal, which is also called coke, and calcium oxide (lime, any kind of lime will not work, it needs to be purified and homogeneous). All this heats up to 2 thousand degrees. And voila, the reaction started.
The result is a liquid substance, which will then become a familiar compound to us. But first it needs to cool in the molds. After the degree is reduced, these layers are crushed into pieces that are more convenient to use.
Now about the silicon version. We got it absolutely by accident, as usually happens. An American scientist tried to create an artificial diamond. As a result of the experiments, carbides were obtained (by the way, they are in second place in hardness after uncut diamonds).
He patented it and opened the first plant for the production of the material. It’s impossible to say that technology has changed a lot since then. Unless sand and salt were excluded from it, what remained was carbon and silica, which are still heated to maximum temperatures in furnaces.
If we talk about the compound with calcium, then for one kilogram you will have to pay about 80 rubles. When it comes to silicon in the composition, throw a couple more rubles on top. The aluminum derivative is also affordable, you will have to spend around a hundred. Titanium, molybdenum and chromium carbide will cost the same amount.
Now about more expensive options, for example, tungsten carbide is not a cheap purchase. Prepare about one and a half thousand rubles, which you will have to say goodbye to when purchasing 1000 grams of raw materials.
There is another “pleasant” bonus: the manufacturer may limit you in choosing the quantity of goods you purchase, because many indicate that you must buy at least 10 kilograms. And if you intend to purchase a composition with boron, then it’s no easier - hardly anyone will sell you less than 30 kilos, while 1 kg. will cost as much as 2 thousand rubles.