There are many different chemical compounds known in the world: about hundreds of millions. And all of them, like people, are individual. It is impossible to find two substances that have the same chemical and physical properties with different compositions.
One of the most interesting inorganic substances existing in the world are carbides. In this article we will discuss their structure, physical and chemical properties, applications and analyze the intricacies of their production. But first, a little about the history of the discovery.
Carbide grade
Carbide class - steel with a high content of carbon and carbide-forming elements;
in the cast state, the structure of such steel contains carbide eutectic; in the deformed state, there are primary and secondary carbides. A typical example of carbide-grade steel is high-speed steel. Carbide grade steel is used to make tools. Due to the high carbon content and a very large number of carbide-forming elements, many carbides are present in its structure. In the cast state, the structure of this steel contains carbide eutectic - ledeburite. The basic structure of carbide-class steel depends on the degree of alloying of austenite, which varies depending on the heating temperature. At low heating temperatures, as soon as the critical point is passed, the dissolution of carbides slows down, and the structure after cooling in air turns out to be sorbite-like with a large amount of excess carbides. Higher heating leads to the dissolution of caribides and the formation of a martensitic structure after cooling in air.
Carbide steels are mostly classified as tool steels.
The carbide class includes steels X12M, P9, P18, etc., containing a large amount of carbon and carbide-forming elements Cr, W, V, etc. This class is characterized by the presence of carbides, while the structure of the main background can be depending on the composition and heating temperatures of pearlite, martensite and austenite. These steels have high hardness and wear resistance. Used for the manufacture of cutting tools and dies operating under difficult conditions.
Carbide grade steel is used mainly for cutting tools.
The structure of carbide class steel (ledeburite) in the heat affected zone.
The structure of carbide class steels in the forged and annealed state consists of sorbitol or granular pearlite, secondary and ledeburite carbides (see Fig. Ledeburite class steels, like pearlitic class steels, are capable of phase transformations and, therefore, can be hardened to martensite. Availability in their structure, a large number of carbides introduces some features into the usual hardening scheme.We will now consider these features.
| Structure of deposited metal when welding pearlitic steel. |
Steels of the carbide class include steels of the martensitic or austenitic class with carbide-forming elements (chrome, tungsten, etc.), due to which the metal structure, along with martensite or austenite, contains a significant amount of carbides.
| Microstructure of wear-resistant high-chromium cast iron (x 250.| Morphology of graphite inclusions in cast iron castings. a - not subjected to modification. modified with UDP in an amount of 0 05% wt. b - TiN SiC Ni, e - SiC Cr. |
In carbide-class steels, UDP additives make it possible to obtain a structure reminiscent of damask steel. The strength of such steel increases by 15-20%, and ductility and impact strength increase by 1-5-2 times, which makes it possible to cast a forging tool with a cast engraving, which is not inferior in properties to a tool made of forged metal.
The most typical steels of the carbide class are high-speed steels. Therefore, we will consider the features of heat treatment of carbide-class steels using the example of high-speed steels, especially since they are of great practical interest.
If alloy steel of the carbide class is forged, then inclusions of carbides are evenly distributed in the main metal mass in the form of globules. Carbide grade steel is used mainly for making tools.
When forging carbide class steel, carbide inclusions are evenly distributed in the main metal mass in the form of globules.
For forgings made of carbide-class steel, which requires the carbides to be crushed and evenly distributed over the cross-section, extensive forging and alternating broaching and upsetting operations are required.
Receipt
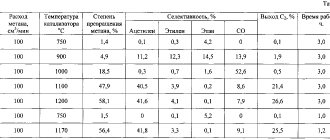
As previously noted, calcium carbide is actively used in the production of a wide variety of materials. That is why the process of obtaining calcium carbide has been constantly improved. The features of the technologies used include the following points:
- Quicklime is used as a raw material. In most cases, the substance is obtained from lime, but it is difficult to carry out such a procedure at home.
- Lime is mixed with crushed coke to obtain a homogeneous mass.
- In industry, calcium carbide is produced according to a scheme that involves heating the substance to a high temperature. Electronic ovens are used for this. The recommended melting point is 1900 ⁰C.
- After heating a substance to such a high temperature, it turns into a liquid state. Special forms are prepared for the work.
When considering how to obtain calcium carbide from carbon, we note that according to established standards, the composition must contain at least 80% of the main substance. The share of impurities should be no more than 25%, which also includes carbon. The production of calcium oxide also releases thermal energy, which is worth considering.
Ionic carbides
Calcium carbide: properties and applications.
obtaining acetylene Salt-like, ionic carbides are formed by the following metals:
- group one metals (the alkali metals);
- group two metals (the alkaline earths);
- group three metals (scandium, yttrium, and lanthanum);
- group 11 metals (copper, silver, and gold);
- group 12 metals (zinc, cadmium, and mercury);
- only aluminum from group 13, (gallium, indium and thallium do not appear to form carbides);
- lanthanides, when forming MC2 and M2C3 carbides (where M is the metal);
- actinides, when forming MC2 and M2C3 carbides.
Most commonly, they are salts of C22− and are called acetylides, ethynides, acetylenediides, or (rarely) percarbides. Some ionic carbides contain other anionic species, such as:
- C4−, sometimes called methanides (or methides) because they hydrolyze to give methane gas;
- C34− ion, sometimes called sesquicarbides, which hydrolyze to give methylacetylene.
The naming of ionic carbides is not consistent and can be quite confusing.
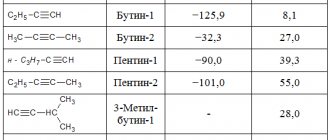
Acetylides
Acetylides contain the polyatomic ion C22−, in which there is a triple bond between the two carbon atoms (similar to acetylene). Examples are carbides of the alkali metals (such as Na2C2), some alkaline earths (such as CaC2) and lanthanoids (such as LaC2).
The CC bond distance ranges from 109.2 picometers (pm) in CaC2 (similar to acetylene), to 130.3 pm in LaC2 and 134pm in UC2.
Methanides
Methanides contain the monatomic ion C4−. Examples of methanides are Be2C and Al4C3.
The C4− ion is a very strong base and will combine with four protons to form methane. The reaction may be written as follows:
C4− + 4H+ → CH4
Methanides commonly react with water to form methane, but reactions with other substances are also common.
Sesquicarbides
The polyatomic ion C34− is found in, for instance, Li4C3 and Mg2C3. The ion is linear and isoelectronic with CO2. The CC distance in Mg2C3 is 133.2 pm. Hydrolysis of Mg2C3 yields methylacetylene (CH3CCH), which was the first indication that it may contain C34−.
Industrial Applications
Calcium carbide is an important compound for the production of acetylene, a gas used in oxy-fuel welding and metal working.
. When burning with oxygen, acetylene can reach 3150 degrees Celsius. This allows you to work with refractory metals that require a temperature twice as high as the melting point of the metal itself.
Boron carbide is used as a refractory material, since the melting point of such a compound is above 2400 degrees. At the same time, it is also found in body armor, as it can protect not only from bullets and shrapnel, but also from radiation. Titanium carbide is used to coat industrial and construction tools. Its strength allows you to increase the wear resistance of parts and process even the most durable materials.
Covalent carbides
Calcium carbide
There are only two carbides that are considered completely covalent; they are formed with the two elements that are most similar to carbon in size and electronegativity, boron (B) and silicon (Si). Silicon carbide (SiC) is known as carborundum and is prepared by the reduction of silicon dioxide (SiO2) with elemental carbon in an electric furnace. This material, like diamond, is extremely hard and is used industrially as an abrasive. It is chemically inert and has a diamond structure in which each silicon atom and each carbon atom are surrounded tetrahedrally by four atoms of the other type. Boron carbide (B4C) has similar properties. It is also extremely hard and inert. It is prepared by the reduction of boron oxide (B2O3) with carbon in an electric furnace. In the structure of B4C, the boron atoms occur in icosahedral groups of 12, and the carbon atoms occur in linear chains of three. Another boron carbide (BC3), which has a graphitelike structure, is produced from the reaction of benzene (C6H6) and boron trichloride (BCl3) at 800 °C (1,500 °F).
References
- Brown Jr., Theodore L., H. Eugene LeMay, Bruce Edward Bursten, and Julia R. Burdge. 2002. Chemistry: The Central Science
. 9th ed. Upper Saddle River, NJ: Prentice Hall. - Chang, Raymond. 2006. Chemistry
. 9th ed. New York, NY: McGraw-Hill Science/Engineering/Math. - Cotton, F. Albert, and Geoffrey Wilkinson. 1980. Advanced Inorganic Chemistry
. 4th ed. New York, NY: Wiley. ISBN 0-471-02775-8. - Ettmayer, Peter, and Walter Lengauer. 1994. Carbides: transition metal solid state chemistry. In Encyclopedia of Inorganic Chemistry
. Editor in chief R. Bruce King. Chichester, UK: Wiley. ISBN 0-471-93620-0. - Greenwood, N. N., and A. Earnshaw. 1998. Chemistry of the Elements
. 2nd ed. Oxford, UK; Burlington, MA: Butterworth-Heinemann, Elsevier Science. ISBN 0750633654. Online version available here Retrieved January 4, 2008.
Constantan alloy - properties and applications
Carbide sludge
Spilling carbide sludge on the territory where the generator is installed, as well as discharging it into sewers or into bodies of untreated wastewater is strictly prohibited. The sludge contains acetylene, soluble in water and adsorbed on the surface of slaked lime, which is gradually released into the atmosphere. In addition, sludge always contains a certain amount of pieces of undecomposed calcium carbide, which, under the influence of water contained in the sludge, atmospheric precipitation and air moisture, decompose, releasing an additional amount of acetylene. The released acetylene may be sufficient to form an explosive mixture with air. This mixture can ignite at the site of sludge drainage, as well as, blown by the wind, in industrial or residential premises. No less dangerous is the ingress of acetylene into air compressors, oxygen installations, etc. In closed sewer lines, an explosion of an acetylene-air mixture can lead to significant destruction.
Discharge of carbide sludge into the general sewer system and wastewater reservoirs without the use of special treatment devices is not permitted.
Various alkali-resistant pigments (kraplak, ultramarine) are sometimes added to carbide sludge and thus not only whiten, but also paint walls and ceilings in various colors.
For whitewashing, carbide sludge is used in the form of diluted lime milk. Whitewashing with lime is possible on both hardened and wet plaster.
Carbide sludge can be used to fill underground spaces and channels in work rooms and laboratories to remove mercury and its vapors. By filling the floors with pulpy carbide sludge and removing it, followed by filling it with a new layer of sludge 10 - 15 cm thick for a long period, you can achieve a significant reduction in the concentration of mercury vapor.
When removing carbide sludge from sludge pits using vacuum tanks, free access of the tanks to the settling tanks must be ensured only in reverse.
To remove carbide sludge from settling tanks, stationary pumps must be provided. It is allowed to use vacuum tankers adapted for pumping and transporting carbide sludge.
| Mobile oxygen dispensing station (Moscow branch of Orgenergostroy.| Volume of sludge settling tank. |
To extract carbide sludge, it is advisable to use a water-jet ejector or a truck crane with a grab.
Considering the ability of carbide sludge to retain acetylene, before repairing devices using heating of their parts, it is necessary to carefully and completely remove sludge from the surfaces of gas generators or other equipment, in order to avoid the danger of the formation of explosive acetylene-air mixtures.
The settling time of carbide sludge depends on the amount of water spent on the decomposition of calcium carbide. The water obtained by settling sludge contains dissolved acetylene; in addition, part of the acetylene is adsorbed by fine sludge particles. When storing sludge, the amount of acetylene it contains gradually decreases. Losses of acetylene with carbide sludge are the main ones during the operation of generators.
Calcium carbide and carbide sludge can cause skin burns and dangerous eye burns.
In the case of using carbide sludge containing 10% Ca (OH) 2, the duration of hydrolysis compared to hydrolysis in pure water increases slightly. When using carbide sludge containing 20% Ca (OH) 2 at 17 C, the hydrolysis process slows down sharply due to silting of the pieces.
The carbide sludge suspension to be filtered was poured into a pressure tank equipped with a heating jacket.
When transporting carbide sludge, the T-158 tank truck should not be equipped with an explosive electrical signaling device to notify the end of filling. You can monitor the filling through the sight glass located in the end bottom of the tank.
Chemical properties
Chemical properties are also important. They are also taken into account when using the material. The main characteristics include the following qualities:
- Calcium carbide is characterized by its ability to absorb moisture well. It is worth considering that such a procedure is manifested by a vivid chemical reaction associated with the decomposition of the substance.
- When working with the material in question, it is worth considering that the resulting dust has an irritating effect on the mucous organs. In addition, a similar reaction can occur when crystals or dust come into contact with the surface of the skin. That is why when working with the compound in question, you should use a respirator and some other protective equipment.
- Crystals actively react to the influence of other substances, often only when heated. This may result in the formation of calcium carbonate.
- In some cases, a crystalline substance is combined with nitrogen, resulting in calcium cyanamide.
- When heated, a reaction can occur with arsenic and chlorine, as well as phosphorus.
Calcium carbonate
The most important chemical quality is considered to be susceptibility to decomposition when exposed to water.
Applications of calcium carbide
As previously noted, calcium carbide is found in a wide variety of industries and is often supplied for industrial synthesis. The properties of calcium carbide and the reaction that occurs when it is combined with various substances determine the use of the substance in the following cases:
- Many synthetic components that make up modern materials are produced on the basis of the component in question.
- Used to produce calcium cyanamide. A similar component is used to produce various chemical fertilizers. That is why raw materials are used to regulate the growth rate of plants.
- Calcium cyanamide is also obtained by combining the substance with nitrogen.
- In some cases, the reduction of alkali group metals is carried out.
- You can use the connection in question in the gas welding process.
When considering calcium carbide and its area of application, it is worth considering that such a substance is most often used to produce acetylene. A similar synthesis of calcium carbide was developed by a German scientist. Among the features of this method of application, we note the following points:
- Acetylene from carbide is obtained by exposing the raw materials used to water.
- As a result of the chemical reaction, the required gas is formed, and slaked lime precipitates.
- It is worth considering that when mixing components, a large amount of heat is generated. Therefore, work must be carried out taking into account safety precautions.
- Depending on the type of raw material processing technology used, about 290 liters of gas come out from 1 kilogram.
- The speed of the procedure depends on the purity of the raw materials used, temperature and amount of water.
Preparation of acetylene from calcium carbide
As practice shows, when using pure carbide, about 20 liters of water per 1 kilogram of raw material are allocated for the chemical reaction. A similar amount of water is required to lower the reaction temperature, thereby ensuring optimal operating conditions.
Physical properties
When choosing almost any material, you should pay most attention to physical properties. For the one under consideration they are as follows:
- The compound has a crystalline structure.
- The melting point is 2300 °C. It is worth considering that such a figure is characteristic only of the pure composition. The addition of various impurities to the composition causes the melting point to drop significantly.
Pure Calcium Carbide
It is worth considering that calcium carbide in most cases is in a solid state. In addition, the color can vary from gray to brown. The physical properties of calcium carbide determine its widespread use in a wide variety of industries.
Hardness.
The process of abrasive processing can be compared with the process of trimming (chisel, chisel, chisel), since the material is removed from the workpiece by the force of sharp protrusions of the abrasive. Therefore, the hardness of the abrasive is a very important parameter. The German mineralogist F. Mohs established the first scale of relative hardness of various minerals in 1820. According to the Mohs scale, the hardness of minerals is estimated from 1 to 10 relative to 10 standards, including talc (1), quartz (7) and diamond (10). The Mohs scale is not uniform, so, for example, the change in hardness when moving from standard 9 to standard 10 is greater than when moving from standard 1 to standard 9.
When evaluating artificial abrasives, it became necessary to expand the Mohs scale.
R. Ridgway added a few numbers to the top end of the scale and changed the position of some of the top Mohs numbers. C. Wooddell measured the degree to which various minerals resist scratching by diamond under controlled conditions and introduced proportionate numbers above the Mohs number 9 (corundum). Knoop hardness numbers are determined by the size of the indentation created when a diamond pyramid is pressed into the material under the influence of a certain load (see table). Table - Various hardness scales
| DIFFERENT HARDNESS SCALES | ||||
| Hardness scale | ||||
| Material | Mohs | Ridgway | Wooddell | Knup |
| Sand | — | 7 | — | 475 |
| Orthoclase | 6 | 6 | — | 560 |
| Quartz | 7 | 8 | 7 | 820 |
| Fused zirconium oxide | 7,5 | 11 | — | 1160 |
| Topaz | 8 | 9 | — | 1250 |
| Pomegranate | 7–7,5 | 10 | — | 1360 |
| Corundum | 9 | — | 9 | 1635 |
| Fused alumina | 9+ | 12 | 10–11 | 2000 |
| Titanium carbide | — | — | — | 2300 |
| Silicon carbide | 9+ | 13 | 13,4–14 | 2450 |
| Boron carbide | 9+ | 14 | 19,7 | 2750 |
| Silicon nitride | — | — | — | 3000 |
| Cubic boron nitride | 9+ | — | — | 4700 |
| Diamond | 10 | 15 | 40–42 | 8000–9000 |
Composition and types of carbides
Carbides are not a separate substance. It is a compound of carbon with metals and non-metals. Moreover, it should be taken into account that carbon must have greater electronegativity in the resulting substance compared to other elements used. This makes it possible to avoid the production of halogens, oxides and other carbon compounds.
Today, there are three types of carbide, the composition of which is different from each other:
- Covalent compounds. This type includes two elements - silicon and bromine. These are compounds with strong interatomic bonds, which ensures a high melting point and chemical inertness. Oxidation of substances in this group is possible only when they are heated above 1000 degrees Celsius. The hardness of the substance containing bromine is so high that it can even compete with diamonds. The substance with silicon is less durable, but has 8 points on the Mohs scale. However, this substance can only be dissolved in aqua regia or using concentrated nitric or hydrofluoric acid.
- Ionic or salt-like compounds. Substances of this group are formed with the help of metals of groups 1 and 2 of the periodic table, as well as aluminum. These compounds are characterized by a high melting point. Ionic carbides decompose under the influence of water and acids. When the reaction occurs, hydrocarbon is released and metal hydroxide remains.
- Ionic covalent metal or metal-like compounds. They are formed with the help of metals from group 4 to 8, as well as cobalt, nickel and iron. A distinctive feature of metal-like substances is their high strength and melting point. This type of connection is divided into two types:
- Acetylenides - upon hydrolysis they form ethylene or acetylene. Calcium carbide belongs to this type of compound.
- Methanides - when reacting with water or dilute acids, they form methane. Most often colorless. These include aluminum, magnesium, and beryllium carbide.
Intermediate transition metal carbides
In these carbides, the transition metal ion is smaller than the critical 135 pm, and the structures are not interstitial but are more complex. Multiple stoichiometries are common. For example, iron forms a number of carbides: Fe3C, Fe7C3, and Fe2C. The best-known of these is cementite (Fe3C), which is present in steels.
These carbides are more reactive than the interstitial carbides. For example, the carbides of Cr, Mn, Fe, Co, and Ni are all hydrolyzed by dilute acids and sometimes by water, to give a mixture of hydrogen and hydrocarbons. These compounds share features with both the inert interstitials and the more reactive, salt-like carbides.
Silica.
Silicon dioxide SiO2 is used in various forms (crystalline, glassy) for shaping and grinding products. Although the different types of silica are chemically identical, they vary widely in physical state, and therefore each has its own specific use.
Diatomaceous earth, diatomaceous earth, diatomaceous earth and tripolite are composed of the siliceous remains of fossilized diatoms. They are used as mild abrasives as components in polishing powders and pastes, such as silver polishing pastes.
Rukhlyak and tripoli are products of the decay of siliceous limestones. They are also used as components of cleaning and polishing powders and pastes.
Crushed quartz, quartzite, flint, chert, sand and sandstone are used in grain form as abrasives in regular sandpaper, as well as in sandblasting and cleaning pastes.
Uncrushed sand with a high quartz content is used for sandblasting and for sawing and grinding soft stone such as marble.
Transportation and storage
Calcium carbide powder decomposes almost instantly when exposed to moisture. This produces acetylene, which in high concentrations is flammable and explosive. That is why it is necessary to pay quite a lot of attention to the storage of calcium carbide, for which cans and special drums are often used. Other storage features include the following:
- The released acetylene is lighter than air, so it accumulates at the top. It is worth considering that it has narcotic effects and can spontaneously ignite.
- When producing large volumes of a substance, special attention is paid to safety precautions. Special packaging is used for packaging.
- To open the package, use tools that do not cause sparks.
- If the substance gets on the skin or mucous membrane, it must be removed immediately. In this case, the affected surface is treated with a special cream or other protective and healing substance.
- According to established rules, transportation can only be carried out using a covered vehicle. However, delivery by air is prohibited.
Transport container
Regulations also prohibit storing calcium carbide with other chemicals and heat sources. This is because the resulting gases can react chemically with other chemicals and ignite.
Safety precautions
Carbide for welding belongs to the class of explosive substances. Safe use of carbide is ensured by taking into account a number of conditions:
- Acetylene is a flammable gas, and dry carbide itself is also explosive, so there should be no open flames near the work site, even small ones such as lighters and lit cigarettes.
- It is prohibited to use carbide in granules up to 2 mm or carbide dust, as it dissolves very quickly, which leads to the release of large amounts of gas. Because of this, ultra-high pressure forms in the generator and an explosion may occur.
- It is prohibited to work with an angle grinder or an electric welding machine near gas welding operations and places where carbide drums are installed.
- The substance must be stored in a dry and sealed place, in which there are no water pipes, sewer pipes and, especially, gas equipment.
- Carbide dust upon contact causes irritation to the skin, eyes, and mucous membranes. Work with this substance must be carried out using personal protective equipment - goggles, gloves and a respirator.
- If carbide gets on your skin or eyes, rinse it with plenty of warm water and then carefully remove any remaining carbide with tweezers or a damp swab.
- When working with carbide in a workshop, all welding equipment must be placed in separate parts of the room, the ventilation system of the room must ensure the removal of flammable gases, and the room must be cleared of flammable materials.
- Acetylene generators are prohibited from being installed in basements and residential buildings.
- Before starting, the generator should be inspected for visible cracks or dents in the housing.
- During work, the generator must remain in a vertical position, the pressure gauge must be in working order and clearly visible to the welder or his assistant.
- At the end of the work, the carbide solution remaining in the generator must be completely exhausted, and the resulting lime must be disposed of.
- Reuse of wet carbide pieces is not permitted;
- Do not open the generator under pressure (during an ongoing reaction).
- Acetylene cylinders are stored and transported with special safety caps on the valves.
If there is a need to regularly use equipment for gas welding and cutting, it is better to buy professional equipment manufactured at an industrial enterprise. The use of homemade generators can result in serious injuries and a threat to life.
How can this be useful in life?
Well, firstly, knowledge of chemical compounds can never be superfluous. It is always better to be armed with knowledge than to be left without it. Secondly, the more you know about the existence of certain compounds, the better you understand the mechanism of their formation and the laws that allow them to exist.
Before moving on to the end, I would like to give some recommendations for studying this material.
Notes
- ↑ Kosolapova T. Ya.
Carbides. - Metallurgy, 1968. - P. 300. - ↑ Tretyakov V.I.
Fundamentals of metal science and technology for the production of sintered hard alloys. - Metallurgy, 1976. - P. 24-268. — 528 p. - Toth L.
Carbides and nitrides of transition metals. - Mir, 1974. - pp. 21-23. — 296 p. - Editorial team: Knunyants I. L. (chief editor).
Chemical encyclopedia: in 5 volumes. - M.: Soviet Encyclopedia, 1988. - T. 1. - P. 420-421. — 623 p. — 100,000 copies. - ↑ Samsonov G.V.
Physical materials science of carbides. - Naukova Dumka, 1974. - P. 79-397. — 454 p. - Kieffer R., Benezovsky F.
Hard alloys. - Metallurgy, 1971. - P. 47. - 392 p. - ↑ Samsonov G.V., Vinitsky I.M.
Refractory compounds (handbook). - Metallurgy, 1976. - P. 560. - Lidin R. A., Molochko V. A., Andreeva L. L.
Chemical properties of inorganic substances. - Chemistry, 2000. - P. 330. - 480 p. - The letter H (German Hartkern) in the designation of German WWII ammunition means “with a solid cermet core.”
- Thus, a 20-mm DM43 BPS, when fired from a MK 20 RH 202 cannon (initial speed 1100 m/s) at a distance of 1000 m, is capable of penetrating 35 mm of steel armor at an impact angle of 0°, but only 8 mm of armor at an angle of 60°. Jane's Infantry Weapons 1996-97, 456.
- Dmitry Safin.
(unavailable link). Compulenta (October 15, 2010). — Prepared from Wiley. Retrieved October 16, 2010.