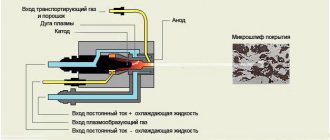
Nickel plating is the process of applying a layer of nickel to the surface of a product. The layer thickness ranges from 1−50 μm. The coatings are black, shiny and matte. They create a reliable surface shell for protection from the environment.
It finds wide application in mechanical engineering, food industry and optics. Nickel plating is carried out on steel, non-ferrous metals: copper, tungsten, aluminum, titanium, as well as plastic.
Description of the method
Nickel coating is formed on various metal structures made of ferrous and non-ferrous metals. Increases corrosion resistance, protects against wear, high humidity, and some chemically active substances.
Nickel coatings are characterized by high hardness, oxidation resistance, and excellent reflectivity.
Coating thickness - from 0.8 to 55 microns. Suitable for application on the following products:
- metal structures, the operation of which is expected in unfavorable atmospheric conditions or in acidic environments;
- vehicle body parts;
- special equipment or instruments that are used in medicine;
- fencing and supporting steel or aluminum structures;
- metal products used in acidic or alkaline environments.
The nickel layer can be matte, glossy or black.
Nickel plating of workpieces made of cadmium, lead, tin, bismuth, and antimony is impossible. Before carrying out work, this feature must be taken into account.
What is nickel plating?
Nickel plating is the process of applying a thin layer of nickel metal to a product to give it the necessary properties.
Coatings are widely used as a sublayer when coating with precious metals, as well as to improve electrical conductivity, increase hardness, protect in alkaline environments and give a highly decorative appearance.
Nickel is a silvery-white metal with a strong luster. The atomic mass of nickel is 58.69 g/mol, density 8.9 g/cm3. It has an electrochemical equivalent of 1.095 g/(A*h), its standard potential is -0.25 V.
Nickel coatings are easily passivated in air and under the influence of strong oxidizing agents. Thanks to this, the coating has high corrosion resistance. With a coating thickness of 125 microns, the base metal is already protected from the effects of industrial gases and solutions. In less aggressive environments, 50-100 microns are sufficient. Nickel is completely stable in alkalis and organic acids of an oxidizing nature.
| Nickel plating designation | N. b - brilliant N - matte Nickel coating designation |
| Thickness | 6-50 microns (greater thickness is also possible) |
| Microhardness | 3420-6900 MPa |
| Electrical resistivity at 18°C | 7.23-10-8 Ohm⋅m. |
| Permissible operating temperature | 650oC |
| Light reflectance | up to 75% |
In a nickel-steel galvanic pair, nickel is the cathodic coating and, therefore, can only provide protection if there are no bare spots or pores. Therefore, it is necessary to obtain coatings with minimal porosity. In a nickel-copper pair, nickel is the anode.
The hardness of nickel obtained from electrolytes without organic additives, which include brightening agents, wetting agents and leveling additives, usually ranges from 3000-4000 MPa. With the introduction of additives, the hardness increases to 6000-7000 MPa. Tensile strength varies accordingly from 60 to 175 kgf/mm2.
Coatings have reduced ductility, but after annealing at 900°C their plastic properties are significantly improved.
There is information about the possibility of using coatings in equipment related to milk processing.
Figure 1 shows the microstructure of the surface of the matte coating on the product.
Figure 1 — Microstructure of the surface of a matte nickel coating.
Processing methods
To coat metal with nickel, you need to choose a method for applying a protective layer. Technologies:
- electrolytic;
- chemical
Chemical nickel plating (Photo: Instagram / chromgoldastra)
Electrolytic method
The nickel plating is applied in an electrolyte bath in which the electrode and workpiece are immersed. A current is passed between the part and the anode, supplied from a laboratory power source or a step-down transformer.
The resulting coating is characterized by high uniformity, a minimum number of defects on the surface, and the absence of pores. Preparing electrolyte at home is quite simple.
Galvanic nickel plating allows you to obtain protective layers with the following characteristics:
- melting point - +1450 degrees;
- Vickers hardness - 250;
- longitudinal type deformation - 10–30%;
- adhesive strength - from 35 to 45 kgf/mm2;
- resistivity -8.510-5 Ohm•m;
- magnetic susceptibility - 37.
Application of a protective layer:
- Prepare a container suitable for the size of the part.
- Place the electrode in the container and place the workpiece on the bracket. It is important that they do not touch the walls of the vessel.
- Pour electrolyte into the container.
- Select a power source whose output produces a voltage of up to 6 V and a current of up to 1.2 A.
- Connect the positive contact of the power source to the anode, and the negative contact to the workpiece.
- Apply voltage to the electrodes.
- The thickness of the applied coating depends on the time the voltage is applied to the electrodes.
- After obtaining a nickel layer of the required thickness, turn off the power source and remove the part.
- If necessary, the coating can be sanded.
Chemical method
The chemical coating method makes it possible to create durable nickel layers on workpieces. It is easy to implement and effective. Does not require any skills or experience in performing such work.
The chemical method is not suitable for applying a protective layer to surfaces with roughness or complex geometry. It is not possible to apply an even layer in hard-to-reach places.
Electroless nickel plating allows you to obtain coatings with the following properties:
- melting point - +8900C;
- Vickers hardness - 550;
- longitudinal type deformation - 3–6%;
- adhesive strength - from 35 to 50 kgf/mm2;
- resistivity - 6010-5 Ohm•m;
- magnetic susceptibility - 4.
Application of a protective layer:
- Prepare a solution for nickel plating by mixing the reagents with water in a container that is resistant to these chemical components.
- Heat the solution until it boils, and then add NaPO2H2.
- Prepare an enameled metal container. Make a dielectric holder. Its design should be such that when lowered into the container the part does not touch the walls.
- Pour electrolyte into the container, lower the workpiece on the bracket.
- Heat the structure to such a temperature that the electrolytic composition boils. Maintain in solution for 1–3 hours, depending on the chemical composition and requirements for coating thickness.
- Remove the finished part and wash it in a slaked lime solution.
- Polish if necessary.
Nickel-plated bolts (Photo: Instagram / galvanika74)
Chemical nickel plating technology
Chemical nickel plating of metal has also become widespread. This technology is also quite simple. The principle of chemical nickel plating is as follows:
- An electrolytic solution is created based on nickel salts with the addition of various additives and sodium hypophosphite.
- The part is placed in the solution, the solution is evenly heated to a temperature of 200-300 degrees, for about 1 hour.
- When the electrolyte is heated, sodium hypophosphite reduces nickel. This leads to the formation of a thin film of nickel on the metal surface.
Please note that the solution does not need to be heated. However, in this case, the nickel film will be very fragile, which will make nickel plating useless. Both acidic and alkaline solutions can be used as an electrolyte. Acid solutions are recommended because they have higher hardness and strength. Also note that the nickel plating format directly depends on what metal the main part is made of.
Baths for treatment
Nickel plating of workpieces is carried out in baths with the addition of:
- Na, Mg or Zn chloride - intended to dissolve the anode material, better reaction in the presence of Zn and other pollutants;
- Nickel sulfate - used as a source of ions for coating;
- boric acid - regulates the acid level in the bath at the required level.
Optimal conditions for chemical reactions to occur:
- composition temperature - +320C;
- acidity pH - from 5.3 to 5.9;
- the amount of nickel sulfate is up to 360 g/l.
Nickel layers with metal surfaces have low bonding strength. Therefore, it is necessary to perform their heat treatment at temperatures up to +4000C, followed by hardening for 3 hours. Exceeding this value will have a negative impact on the properties of the metal. The optimal range is from +2600С to +3100С.
Special mixing equipment is installed inside the bathroom to achieve uniformity of the solution. Filters are used to remove various contaminants.
Productive baths for industrial use require the addition of foam suppressants or compressed air.
Carrying out electrolytic nickel plating at home
Electrolytic (galvanic) nickel plating of parts is carried out in two ways:
- immersing parts in electrolyte;
- without immersing parts in electrolyte.
The first method is used when processing small-sized parts. The second method is used when processing large and heavy objects.
Before nickel plating, the metal is copper-plated.
Copper plating of metal has a general definition such as electroplating
Electrolyte immersion method
Main stages of the process
According to the first method, the surface of the product is sanded with sandpaper to remove the oxide film. The sample is then washed in warm water. After this, it is treated with a soda solution and washed again in warm, clean water.
Then two thin copper plates are placed in a glass or porcelain dish. The plates act as anodes. They are placed in a vertical position, parallel to each other.
The product is placed between these two plates. To do this, the sample is suspended using a wire. The wire is attached to the plates at both ends.
An aqueous electrolyte solution with the following composition is added to the dishes:
- distilled water;
- 20% copper sulfate;
- 2% sulfuric acid.
The copper plates are connected to a power source. The voltage value is determined at the rate of 15–20 mA per 1 cm2 of the surface of the material.
For your information. Nickel electrolyte is sensitive to changes in acidity. To maintain the acidity level, buffer compounds based on boric acid are used.
In an electrolyte solution, copper chloride dissociates (breaks up) into its constituent components. Copper ions are displaced towards the cathode and turn into neutral atoms. Chlorine ions are oxidized at the anode.
When current is passed through an electrolyte, copper ions pass into solution. From solution, copper settles on the cathode in the form of neutral atoms. Impurities remain at the bottom of the pan. The purity of the resulting copper is almost 100%.
After 30 minutes, a thin layer of copper will form on the part. Exposure to electric current causes an increase in the thickness of the copper layer. The greater the thickness of the layer, the fewer pores remain on the treated surface.
Method without immersing parts in electrolyte
Electroplated nickel plating
Galvanic nickel plating of large parts is carried out without immersing them in an electrolyte. To do this, use a brush made of loose copper wires. Multicore copper cable stripped of insulation is often used as a brush.
By increasing the deposited copper layer, the porosity of the sample surface is eliminated.
The nickel deposition process is carried out similarly to the surface copper plating process. To do this, add electrolyte to the container. The electrolyte contains the following chemical reagents, g/l:
- sodium sulfate solution – 310;
- nickel chloride solution – 65;
- orthoboric acid – 45;
- 1,4-butanediol – 0.15;
- ortho-sulfobenzimide (saccharin) – 2.0;
- kaolin (lime) – 1.0.
Thin nickel plates are dipped into the electrolyte. They play the role of anodes. The product is placed between them. The ends of the plates are connected to the terminal of the power source with a positive charge. The body of the part is connected to the negative pole.
A rheostat is used to regulate the current value. The amount of supplied electric current is monitored using a milliammeter. The magnitude of the supplied current should not exceed 6 V. Nickel deposition is carried out at a temperature of about 50°C and an electric current density of 4–5 A/dm2. The duration of the process is 3 minutes.
For your information. Nickel coating without a substrate has rather weak adhesion to the surface. In order to increase adhesion, heat treatment of the product is used at a temperature of 450 degrees.
The final stage of part processing
The treated part is washed under a stream of clean warm water and dried.
The nickel-plated finish has a matte finish. The part is polished to add shine.
Nickel coatings with defects are removed by anodic dissolution in an electrolyte. To do this, sulfuric acid is included in the electrolyte. The chemical density of the acid is taken to be 1.2-2.8 kg/m3. The process of removing the nickel layer is carried out at a temperature of 20-25 ° C and an anodic electric current density of 5 A/dm2.
Product preparation
The process of nickel plating at home requires proper preparation:
- using abrasive tools, the oxide film or surface layer of rusty metal is removed;
- use a brush to remove sawdust from the surface of the workpiece;
- wash away traces of dirt, if any, with water;
- the workpiece is degreased with a solvent or soda composition;
- Traces of degreasers are washed off with water and then dried.
Abrasive material (Photo: Instagram / to4kacveta)
In what cases is nickel plating used?
- Due to the strength of the coating and its high protective properties, nickel plating has found application in many areas:
- Construction
– to protect metal structures used outdoors or in water. - Mechanical engineering
– for coating and restoration of parts of cars and motor vehicles, including those made of aluminum alloy. - Medicine
– in the production of medical and dental instruments. - Chemical industry
- to protect metal products exposed to reagents. - Production of consumer goods
- to protect and impart high aesthetic properties to plumbing fixtures, household tools, as well as interior items. In this area, nickel plating of non-metallic surfaces is practiced: glass, polymer, ceramic, etc.
Application of a protective layer
To perform nickel plating on steel, a layer of copper must be applied to its surface. Carrying out work:
- Select a glass container.
- Prepare electrolyte. To do this, you need to prepare 20% CuSO4, 2% H2SO4, 78% H2O.
- Place the workpiece in a container. Place anodes on opposite sides.
- Assemble an electrical circuit based on a laboratory current source. Its power is determined based on the nickel plating area - if you need to process 10–15 mA/cm2 of the surface of the part, the voltage should be in the range from 5.8 to 6 V.
- Apply voltage to the connected circuit. The optimal coating thickness is achieved within 30 minutes.
You can also apply the protective layer with a brush:
- A brush is made from stranded copper wire by removing the insulating layer. For convenience, it is fixed on a wooden block with a cross-section convenient for work.
- The workpiece is cleaned, degreased, and then washed from solvents.
- Take a laboratory power source. A brush is connected to the positive contact, and a workpiece is connected to the negative contact.
- Prepare an electrolyte solution.
- Turn on the power to the current source.
- The brush is immersed in an electrolytic solution, then removed and passed over the workpiece.
- Consistently and evenly cover the entire workpiece with copper coating. The thickness is determined by the time of exposure to a certain surface area.
Influence of operating conditions of coatings on their properties.
Figure 11 — Effect of operating temperature on tensile strength, yield strength and elongation of electrodeposited nickel.
Figure 12 - Effect of deposit thickness and internal stresses on the 100 million cycle fatigue life of nickel-plated AISI 4340 stainless steel.
How to increase the durability of the coating?
To increase the resistance of nickel-plated coatings to negative influences, it is necessary:
- coat the part with copper to reduce roughness;
- pour a solution of MgO and H2O and HCl or H2SO4 into the installation for chemical nickel plating;
- apply a deep penetration lubricant, then immerse the part in a container with purified fish oil, take it out and remove the remaining composition;
- apply multilayer nickel plating;
- Perform fish oil treatment twice with an interval of 12 hours.
When is heat treatment required?
If the workpiece needs to ensure the qualities of wear resistance and hardness, a heat treatment operation is performed. The increase in these properties is due to the fact that, under conditions of increasing temperature, a nickel-phosphorus deposit occurs, followed by the formation of a new chemical compound. It helps to increase the hardness in the coating structure.
Depending on the temperature regime, microhardness changes with different characteristics. Moreover, the correlation is not at all uniform with respect to an increase or decrease in heating temperature. When heat treated as part of chemical nickel plating at conditions of 200 and 800°, for example, the microhardness indicator will be only 200 kg/mm2. The maximum hardness value is achieved at temperatures of 400-500°. In this mode, you can count on providing 1200 kg/mm2.
It should also be borne in mind that heat treatment is not generally permissible for all metals and alloys. For example, a ban is imposed on steels and alloys that have already been subjected to hardening and normalization procedures. It is worth adding to this the fact that heat treatment in air can contribute to the formation of a tarnished color, changing from golden to purple. Reducing the temperature to 350° will help minimize such factors. The entire process is carried out in about 45-60 minutes only with the workpiece cleaned of contaminants. External polishing will directly affect the likelihood of obtaining a high-quality result.
Removing the coating
You can remove the nickel-plated layer in the following ways:
- electrochemical etching in a 30% H2SO4 solution;
- mechanical processing with abrasive tools;
- sandblasting.
To avoid removing the metal layer from the substrate when adding acid, you need to add glycerin to the solution in an amount of 50 g/l.
The nickel layer can protect the metal from oxidation, serve a decorative role, or serve as a substrate for chrome plating. The technology is easy to implement and does not require expensive equipment or special education.
Definition
Nickel plating is a set of procedures that results in the creation of a thin film of nickel on the surface of a metal alloy. The film layer is quite small - from 1 to 50 micrometers, and the thickness of the nickel layer can be controlled by changing the proportion of nickel in the electrolytic solution. Nickel plating of metal is used to improve the original physical characteristics of the base metal alloy:
- Increased corrosion resistance. Nickel is highly chemically inert, so it does not come into contact with oxygen and water. Therefore, nickel will prevent corrosion on the surface of the metal element.
- Protection against weak acids and alkalis. Nickel also withstands weak chemicals well, so it can also be used to create an additional layer that will protect the base material from acids and alkalis.
- Creates a durable outer covering. When mechanical damage occurs, the appearance of the metal product changes, and its technical and operational characteristics may also deteriorate. Creating an additional layer of nickel is advantageous because if the metal is damaged, you can always quickly apply a new layer.
- Nickel has a pleasant silver-gray sheen, so nickel plating can also be done for decorative purposes. Decorating metal toys, creating beautiful nickel-plated frames, and so on).
Almost any metal can be nickel-plated - steel, cast iron, various iron alloys, copper, brass, aluminum, titanium, and so on. Processing objects include solid sheets, parts with holes, plumbing fixtures, bolts, screws, fish hooks, and so on.
There are two technologies - galvanic and chemical nickel plating. Both technologies are widely used in factory production. If necessary, you can do the treatment at home yourself.