Image taken from Popular Mechanics website
Many have seen an experience with a permanent magnet that seems to get stuck inside a thick-walled copper tube. In this article we will understand the physics of the process. First, we write the formula for the magnetic field of a permanent magnet, and calculate what magnetic flux passes through the cross section of the pipe, then we make the magnet move and find out what induced electric current occurs in the metal, what is the dissipated electrical power, we write and solve the equation of motion of the permanent magnet.
And if you have read this far and are not scared, welcome to the cut - it will be more interesting further! I myself have been thinking for a long time about taking a good look at this issue. And recently I had a conversation with a work colleague. His child was assigned to do a science demonstration at school, for which his dad got hold of a piece of copper pipe and a neodymium-iron-boron magnet. The child figured it out, demonstrated the experiment in front of the class, gave explanations, but neither the class nor the teacher were particularly impressed. A volcano (!) made from soda and citric acid won the competition of scientific experiments =) My colleague and I figured it out in words and realized that the matter was clear, that the matter was dark. And not much has been written in the literature on this topic. This conversation inspired me to try to get through the jungle. In this article I write what I did.
Scientific point of view
To determine which metals are not magnetic, you need to find out how all metals in general can relate to magnets and a magnetic field. With respect to the applied magnetic field, all substances are divided into diamagnetic, paramagnetic and ferromagnetic.
Each atom consists of a positively charged nucleus and negatively charged electrons. They move continuously, which creates a magnetic field. The magnetic fields of electrons in one atom can enhance or cancel each other, depending on the direction of their movement. Moreover, the following can be compensated:
- Magnetic moments caused by the movement of electrons relative to the nucleus are orbital.
- Magnetic moments caused by the rotation of electrons around their axis are spin moments.
If all magnetic moments are equal to zero, the substance is classified as diamagnetic. If only spin moments are compensated - to paramagnets. If the fields are not compensated, use ferromagnets.
Magnetic properties
Each atom has a quantity called a total magnetic moment, which is determined by the movement of electrons along their orbit. The magnetic moment determines the amount of susceptibility of a substance to a magnetic field. All metals are divided into three groups:
- Diamagnets are substances with negative magnetic susceptibility, i.e. they do not magnetize. These include: zinc, gold, copper and others.
- Paramagnetic materials have a positive magnetic susceptibility value, but not high. These are magnesium, platinum, chromium, aluminum and others. They are magnetic, but weakly.
- Ferromagnets are substances that have a strong susceptibility to a magnetic field. These include: nickel, cobalt, iron, some rare earth metals, iron alloys and others.
Copper in the periodic table
Paramagnets and ferromagnets
Let's consider the option when each atom of a substance has its own magnetic field. These fields are multidirectional and compensate each other. If you place a magnet next to such a substance, the fields will be oriented in one direction. The substance will have a magnetic field, a positive and a negative pole. Then the substance will be attracted to the magnet and can itself become magnetized, that is, it will attract other metal objects. For example, you can magnetize steel clips at home. Each one will have a negative and a positive pole, and you can even hang a whole chain of paper clips on a magnet. Such substances are called paramagnetic.
Ferromagnets are a small group of substances that are attracted to magnets and are easily magnetized even in a weak field.
Diamagnets
In diamagnetic materials, the magnetic fields inside each atom are compensated. In this case, when a substance is introduced into a magnetic field, the movement of electrons under the influence of the field will be added to the natural movement of electrons. This movement of electrons will cause an additional current, the magnetic field of which will be directed against the external field. Therefore, the diamagnetic material will be weakly repelled from the nearby magnet.
So, if we approach the question from a scientific point of view, which metals are not magnetic, the answer will be – diamagnetic.
Distribution of paramagnets and diamagnets in the periodic table of Mendeleev elements
The magnetic properties of simple substances change periodically with increasing atomic number of the element.
Substances that are not attracted to magnets (diamagnets) are located mainly in short periods - 1, 2, 3. Which metals are not magnetic? These are lithium and beryllium, and sodium, magnesium and aluminum are already classified as paramagnetic.
Substances that are attracted to magnets (paramagnets) are located mainly in the long periods of the Mendeleev periodic system - 4, 5, 6, 7.
However, the last 8 elements in each long period are also diamagnetic.
In addition, three elements are distinguished - carbon, oxygen and tin, the magnetic properties of which are different for different allotropic modifications.
In addition, there are 25 more chemical elements whose magnetic properties could not be established due to their radioactivity and rapid decay or the complexity of synthesis.
The magnetic properties of lanthanides and actinides (all of which are metals) change irregularly. Among them there are para- and diamagnetic materials.
There are special magnetically ordered substances - chromium, manganese, iron, cobalt, nickel, the properties of which change irregularly.
Is Copper Magnetic? - Metalist's Handbook
February 24, 2015.
In the magnetic circuits of various electrical machines, transformers, instruments and apparatus of electrical engineering, radio engineering and other branches of technology, a variety of magnetic and non-magnetic materials are found.
The magnetic properties of materials are characterized by the values of magnetic field strength, magnetic flux, magnetic induction and magnetic permeability.
The relationship between magnetic induction and magnetic field strength, expressed graphically, forms a curve called a hysteresis loop. Using this curve, you can obtain a series of data characterizing the magnetic properties of the material.
An alternating magnetic field causes the appearance of eddy currents in magnetic materials. These currents heat the cores (magnetic cores), which leads to the consumption of some power.
To characterize a material operating in an alternating magnetic field, the total value of power expended on hysteresis and eddy currents at a frequency of 50 Hz is referred to 1 kg of material weight. This value is called specific losses and is expressed in W/kg.
The magnetic induction of a particular magnetic material should not exceed a certain maximum value, depending on the type and quality of the material. Attempts to increase induction lead to increased energy losses in a given material and its heating.
Magnetic materials are classified as soft magnetic and hard magnetic.
Magnetic soft materials
Soft magnetic materials must meet the following requirements:
- have a large relative magnetic permeability µ, which makes it possible to obtain a large magnetic induction B with the smallest possible number of ampere-turns;
- have the lowest possible losses due to hysteresis and eddy currents;
- have stable magnetic properties.
Soft magnetic materials are used as magnetic cores of electrical machines, transformer cores, chokes, relay electromagnets, electrical measuring instruments, and the like. Let's look at some soft magnetic materials.
Electrical hardware
obtained by electrolysis of sulphide or ferric chloride, followed by melting in vacuum of the electrolysis products. Powdered electrolytic iron is used for the production of magnetic parts, similar to the production of ceramics or plastics.
Carbonyl iron
obtained in the form of a powder as a result of the thermal decomposition of a substance that includes iron, carbon and oxygen [Fe(CO)5].
At a temperature of 1200 °C, carbonyl iron powder is sintered and used to manufacture the same parts that are made from electrolytic iron. Carbonyl iron is characterized by high purity and ductility; used in the electrovacuum industry, as well as in instrument making for the manufacture of laboratory instruments and devices.
The two types of highly pure iron we considered (electrolytic and carbonyl) contain no more than 0.05% impurities.
Electrical steel sheets
is the most common material in electrical engineering and transformer manufacturing. Electrical steel is alloyed with silicon to improve its magnetic properties and reduce hysteresis losses. In addition, as a result of the introduction of silicon into the steel composition, its resistivity increases, which leads to a decrease in eddy current losses.
Sheet thickness depending on the steel grade is 0.3 and 0.5 mm. Electrical steel, cold rolled and then annealed in a hydrogen atmosphere, has particularly high magnetic properties. This is explained by the fact that the metal crystals are located parallel to the rolling direction. This steel is designated by the letters KhVP (cold-rolled high permeability, textured).
Steel sheets have dimensions from 1000 × 700 to 2000 × 1000 mm.
The grades of electrical steel used to be designated, for example, as follows: E3A, E1AB, E4AA. The letter E means electrical steel; letter A – reduced power losses in an alternating magnetic field; letters AA - especially low losses; letter B – increased magnetic induction; numbers 1 – 4 show the amount of silicon contained in steel as a percentage.
According to GOST 802-54, new designations for electrical steel grades have been introduced, for example: E11, E21, E320, E370, E43. Here the letter E stands for electrical steel; first numbers: 1 – lightly doped with silicon; 2 – medium doped with silicon; 3 – highly alloyed with silicon and 4 – highly alloyed with silicon.
The second digits in the designation of grades indicate the following guaranteed magnetic and electrical properties of steels: 1, 2, 3 – specific losses during magnetization reversal of steels at a frequency of 50 Hz and magnetic induction in strong fields; 4 – specific losses during magnetization reversal of steels at a frequency of 400 Hz and magnetic induction in average fields; 5, 6 – magnetic permeability in weak fields (H less than 0.01 A/cm); 7, 8 – magnetic permeability in medium fields (H from 0.1 to 1 A/cm). The third digit 0 indicates that the steel is cold-rolled, textured.
Permalloy
an alloy of iron and nickel. Approximate composition of permalloy: 30–80% nickel, 10–18% iron, the rest copper, molybdenum, manganese, chromium. Permalloy is easily processed and is available in sheet form.
It has very high magnetic permeability in weak magnetic fields (up to 200,000 H/cm).
Permalloy is used for the manufacture of telephone and radio communication parts, transformer cores, inductors, relays, and parts of electrical measuring instruments.
Alsifer
an alloy of aluminum, silicon and iron. The approximate composition of alsifer is: 9.5% silicon, 5.6% aluminum, the rest is iron. Alsifer is a hard and brittle alloy, so it is difficult to process.
The advantages of alsifer are high magnetic permeability in weak magnetic fields (up to 110,000 H/cm), high resistivity (ρ = 0.81 Ohm × mm²/m), and the absence of scarce metals in its composition.
Used for the manufacture of cores operating in high-frequency installations.
Permendur
an alloy of iron with cobalt and vanadium (50% cobalt, 1.8% vanadium, the rest iron). Permendur is available in the form of sheets, strips and tapes. It is used for the manufacture of electromagnet cores, dynamic loudspeakers, membranes, telephones, oscilloscopes and the like.
Magnetodielectrics
These are magnetically soft materials, crushed into small grains (powder), which are isolated from one another by resins or other binders. Electrical iron, carbonyl iron, permalloy, alsifer, magnetite (feO Fe2O3 mineral) are used as magnetic material powder.
Insulating binders are: shellac, phenol-formaldehyde resins, polystyrene, liquid glass and others. The magnetic material powder is mixed with an insulating binder, thoroughly mixed, and from the resulting mass the cores of transformers, chokes, and radio equipment parts are pressed under pressure.
The granular structure of magnetodielectric materials causes low losses due to eddy currents when these materials operate in magnetic fields of high-frequency currents.
Hard magnetic materials
Hard magnetic materials are used to make permanent magnets. These materials must meet the following requirements:
- have a large residual induction;
- have a high maximum magnetic energy;
- have stable magnetic properties.
The cheapest material for permanent magnets is carbon steel (0.4 - 1.7% carbon, the rest is iron). Magnets made of carbon steel have low magnetic properties and quickly lose them under the influence of heat, shock and shock.
Alloy steels have better magnetic properties and are used for the manufacture of permanent magnets more often than carbon steel. These steels include chromium, tungsten, cobalt and cobalt-molybdenum.
For the manufacture of permanent magnets, alloys based on iron - nickel - aluminum have been developed in technology. These alloys are characterized by high hardness and brittleness, so they can only be processed by grinding. The alloys have exceptionally high magnetic properties and high magnetic energy per unit volume.
Table 1 shows data on the composition of some hard magnetic materials for the manufacture of permanent magnets.
Table 1
Chemical composition of magnetically hard materials
| Name of material | Chemical composition in weight percent | Relative weight per unit magnetic energy |
| Carbon steel Chromium steel Tungsten steel Cobalt steel Cobalt-molybdenum steel Alni Alnisi AlnicoMagnico | 0.45 C rest Fe 2 – 3 Cr; 1 C 5 W; 1 C 5 – 30 Co; 5 – 8 Cr; 1.5 – 5 W 13 – 17 Mo; 10 – 12 Co 12.5 Al; 25 Ni; 5 CH 14 Al; 34 Ni; 1 Si 10 Al; 17 Ni; 12Co; 6 CH24 Co; 13 Si; 8 Al; 3 Dc | 26,7 17,2 15,8 5,1 – 12,6 3,8 3,6 3,4 3,11 |
What metals are not magnetic: list
There are only 9 ferromagnets, that is, metals that are highly magnetic, in nature. These are iron, cobalt, nickel, their alloys and compounds, as well as six lanthanide metals: gadolinium, terbium, dysprosium, holmium, erbium and thulium.
Metals that are attracted only to very strong magnets (paramagnetic): aluminum, copper, platinum, uranium.
Since in everyday life there are no such large magnets that would attract a paramagnetic material, and also no lanthanide metals are found, we can safely say that all metals except iron, cobalt, nickel and their alloys will not be attracted to magnets.
So, what metals are not magnetic to a magnet:
- paramagnetic materials: aluminum, platinum, chromium, magnesium, tungsten;
- diamagnetic materials: copper, gold, silver, zinc, mercury, cadmium, zirconium.
In general, we can say that ferrous metals are attracted to a magnet, non-ferrous metals are not.
If we talk about alloys, then iron alloys are magnetic. These primarily include steel and cast iron. Precious coins can also be attracted to a magnet, since they are not made of pure non-ferrous metal, but of an alloy that may contain a small amount of ferromagnetic material. But jewelry made of pure non-ferrous metal will not be attracted to a magnet.
What metals do not rust and are not magnetic? These are ordinary food grade stainless steel, gold and silver items.
Alloys and their magnetic properties
Copper is not magnetic. If you still come across a coin that looks like copper, but has magnetic properties, then most likely it is an alloy. In such an alloy there will be no more than 50% copper. This can be done on purpose, but there have been cases when copper exhibited magnetic properties that was not cleared of impurities during the coin making process.
Every person needs at least minimal knowledge about the magnetic properties of metals. In most cases, this is enough to determine copper - the copper product will not stick to the magnet.
Any child knows that metals are attracted to magnets. After all, they have more than once hung magnets on the metal door of the refrigerator or letters with magnets on a special board. However, if you put a spoon against a magnet, there will be no attraction. But the spoon is also metal, so why does this happen? So, let's find out which metals are not magnetic.
Magnet, copper pipe and Foucault currents
We continue to study physical phenomena and unusual effects with magnets.
Many even fully grown people do not understand the connection between magnetism and electricity. Meanwhile, this connection underlies almost all modern electrical engineering - from generators to electric motors. And the easiest way to show it is with a regular magnet and a copper pipe.
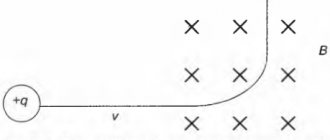
For the experiment you will need only two things - a neodymium magnet and an ordinary metal pipe made of a non-magnetic material, for example, copper. The inner diameter of the pipe should be slightly larger than the magnet itself. Well, now try just dropping the magnet on the floor - outside the pipe for the first time. And a second time, into the pipe.
In the first case, the magnet will simply fall to the floor in about a second. Now pick up the magnet from the floor and throw it inside the pipe. Keep the pipe vertical. And while you are waiting for a magnet to appear from the lower section of a completely non-magnetic (but definitely conductive!) pipe, let’s try to explain why this takes so much time. If we look inside the pipe, we will see that the magnet slowly, as if floating, goes down.
The reason for this is the inextricable connection between magnetism and electricity. The movement of the magnet generates a change in the magnetic field, which, in turn, induces circulating circular (eddy) currents in the pipe.
And these currents generate magnetic fields that interact with the magnet’s field, slowing down its fall. Well, now you know the reason, and you can show your friends a spectacular trick. More precisely, you can do this when the magnet finally flies the pipe to the end.
What kind of currents are these?
Eddy currents
, or
Foucault currents
(in honor of J. B. L. Foucault) are eddy induction currents that arise in conductors when the magnetic field penetrating them changes.
Foucault currents arise under the influence of an alternating electromagnetic field and, by their physical nature, are no different from induction currents arising in linear wires. They are vortex, that is, they are closed in a ring. The electrical resistance of a massive conductor is low, so Foucault currents reach very high strength.
How to distinguish copper from other metals
For most of us, knowledge about copper and its properties is limited to a school chemistry course, which is quite enough at the everyday level.
However, sometimes there is a need to reliably determine whether a material is a pure element, an alloy, or even a composite material.
The opinion that this information is needed only by those who are engaged in the acceptance or delivery of scrap metal is erroneous: for example, on amateur radio forums, topics are often raised about how to distinguish copper in wires from copper-plated aluminum.
Briefly about element No. 29
Pure copper (Cu) is a golden-pink metal with high ductility, thermal and electrical conductivity. Chemical inertness in an ordinary non-aggressive environment is ensured by a thin oxide film, which gives the metal an intense reddish tint.
The main difference between copper and other metals is color.
. In fact, there are not so many colored metals: only gold, cesium and osmium are similar in appearance, and all elements included in the group of non-ferrous metals (iron, tin, lead, aluminum, zinc, magnesium and nickel) have a gray color with varying intensity of shine.
An absolute guarantee of the chemical composition of any material can be obtained only through spectral analysis. The equipment for carrying it out is very expensive, and even many expert laboratories can only dream of it. However, there are many ways to distinguish copper at home.
with a high degree of probability.
Determination by color
So, we have before us a piece of unknown material that needs to be identified as copper. The emphasis on the term “material” rather than “metal” was made specifically, since recently many composites have appeared that are very similar to metals in appearance and tactile sensations.
First of all, we consider color. It is advisable to do this in daylight or “warm” LED lighting (under “cool” LEDs, the reddish tint changes to yellow-green). It is ideal if there is a copper plate or wire for comparison - in this case, errors in color perception are practically eliminated.
Important: old copper products can be covered with an oxidized layer (a greenish-blue loose coating): in this case, the color of the metal must be looked at in a cut or saw cut.
Determination by magnet
Color matching is a reliable but not sufficient method of identification. The second step of independent experiments will be a test with a magnet. Chemically pure copper is classified as diamagnetic - i.e. to substances that do not respond to magnetic influence.
If the material under study is attracted to a magnet, then it is an alloy in which the content of the main substance is no more than 50%.
However, even if the sample did not react to the magnet, it is too early to rejoice, since often an aluminum base is hidden under the copper coating, which is also not magnetic (this can be eliminated by filing or cutting).
Determination by reaction to flame
Another way to identify copper is to heat a sample over an open fire (gas stove, lighter or regular match). When heated, copper wire will first lose its shine and then turn black-brown, covered with oxide. This method can also be used to cut off composite materials that, when heated, begin to smoke and form a gas with a pungent odor.
Determination through chemical experiments
The reaction with concentrated nitric acid is indicative: if the latter is dropped onto the surface of a copper product, a green-blue color will occur.
A qualitative reaction to copper is dissolution in hydrochloric acid followed by exposure to ammonia. If a copper sample is left in an HCl solution until completely or partially dissolved, and then ordinary pharmaceutical ammonia is dropped into it, the solution will turn intensely blue.
Important: working with chemicals requires precautions. Independent experiments should be carried out in a well-ventilated area using personal protective equipment (rubber gloves, apron, goggles).
How to distinguish between copper and its alloys?
Copper alloys are widely used in industry.
Over many years of research, it has been possible to obtain many materials with unique properties: high ductility, electrical conductivity, chemical resistance, strength (all depends on alloying additives).
The most common are bronze (with the addition of tin, aluminum, silicon, manganese, lead and beryllium), brass (with the addition of 10-45% zinc), as well as copper-nickel alloys (nickel silver, cupronickel, copel, manganin).
Only bronze and brass are difficult to identify, since copper-nickel alloys differ significantly in color due to their low copper content.
Copper or brass?
Brass can contain from 10 to 45% zinc, a silver-gray metal. Naturally, the more zinc, the paler the alloy. However, high-copper brasses, in which the amount of additives does not exceed 10%, differ little in color from the copper sample.
In this case, you can only trust your feelings: brass is much harder and more difficult to bend (for greater reliability, a comparison with a reference sample is advisable). You can try to remove the shavings: copper shavings will have a curl shape, brass shavings will be straight, needle-shaped.
When the samples are placed in a solution of hydrochloric acid, no reaction with copper is observed, and a white coating of zinc chloride forms on the surface of the brass.
Copper or bronze?
Like brass, bronze is much stronger, which is explained by the presence of harder metals in the alloy. The most reliable test will be a “tooth test” - there is unlikely to be a trace of pressure left on the surface of the bronze.
You can also experiment with a hot saline solution (200 g of table salt per 1 liter of water). After 10-15 minutes, a copper sample will acquire a more intense shade than a bronze one.
For those familiar with electrical engineering
Very often, copper cores from electrical cables are sold as scrap non-ferrous metals, and there are often cases when copper-coated aluminum is used in the production of electrical products. This material has a significantly lower density, but due to its irregular geometric shape, determining the volume to calculate the density is quite difficult.
In this case, copper can be determined by electrical resistance (of course, if you have the appropriate instruments - a voltmeter, ammeter, rheostat). We measure the cross-section and length of the core, take instrument readings, and Ohm’s law will help you.
Resistivity is a fairly accurate characteristic by which any metal can be identified with a high degree of reliability.
Conclusion
It is possible to accurately determine the quality of copper scrap or the content of the main substance in the alloy only after an examination: all of the above methods are approximate.
If we consider pricing when purchasing scrap metal, the most expensive is electrical copper, the cheapest are alloys of the brass group.
The final cost of the transaction can be clarified with the managers of companies involved in the purchase of scrap non-ferrous metals.
Are there search magnets for gold, silver, copper? (answer: NO)
Only steels have magnetic properties , and not all of them. For example, austenitic stainless steels do not attract magnets because they do not have ferromagnetic properties. However, there are a sufficient number of enthusiasts who believe that magnetic waves are emitted by any metal, and therefore there should be a search magnet for gold and silver, and for some this expression is quite normal for perception and practical use.
ATTENTION! MAGNETS FOR SEARCHING GOLD, COPPER, SILVER DO NOT EXIST!
THEY SIMPLY ARE NOT - ANYWHERE!
In our article we describe the theory of how non-ferrous and precious metals can be detected using magnetic fields. This article is our fantasy, supported by scientific developments of foreign scientists.
See also the article - Extraction of scrap metal from water (about ferrous metal and search magnet).
Equation of motion
Now it's time for the equation of motion.
Using Newton's second law, it will be very easy to write it down. Solving the equation for is not interesting, because, well, the coordinate simply changes at a constant speed. It is much more useful to know how quickly the fall stabilizes, what the steady-state speed of fall is. In general, we need to solve this equation for speed. And the solution will be like this. Here is the damping coefficient. The characteristic time for reaching a steady state of decline is . Initial speed - , steady speed - . In general, this is the equation of a parachutist. This is probably why the Popular Mechanics article is called “Magnetic Parachute”.
Device for adjusting the magnetic field from metal objects
Strictly speaking, this is not a magnet, but rather an electromagnet, with the help of which you can initiate and configure any magnetic radiation, even quite weak ones, to be captured by appropriate devices. It is not easy to build such a device, but the authors, citizens of Australia, have no doubt about its effectiveness. That's why they patented their invention in their patent office. Based on the fact that Australian soil is not much different from domestic soil, we will give a description of the device and operating principle of such a magnet for gold and silver. Although it is necessary to repeat - in the generally accepted sense, this design has nothing .
The operation of the device is based on the well-known physical fact that when any object that generates magnetic oscillations in an alternating electric field moves, changes occur inside the trapper circuit associated with the movement of atoms around the nucleus. If the area of electric field generation is sequentially moved along or across the magnetic field from a metal object, changes will occur in this area, the intensity of which determines the degree and strength of the interaction of two fields - magnetic and electric.
The difficulty is that strong magnetic fields are not created by noble metals . It is known, for example, that, according to the principle of decreasing, the electrochemical potentials of non-ferrous metals are located as follows (we consider only the area of interest to us): copper → mercury → silver → palladium → platinum → gold. Thus, if the expression “is copper attracted to a magnet” may still have some basis, then the phrase “magnet for gold” does not make any sense at all. It is more correct to talk about an electromagnetic trap, which will record the fact of a coordinated change in electric and magnetic fields in a certain, rather local, metallic volume.
Video - how copper interacts with a magnet:
Recording of changes that occur in the apparatus under the influence of such fields is captured by the measuring circuit. It is a highly sensitive spring made of rhenium, a rare metal that is absolutely insensitive to temperature changes. The rhenium spring must be adjusted to operate. The process is to set the conditional zero of the device, for which it is placed as far as possible from all metal objects. In urban areas, such a “search magnet for gold, silver and other precious metals” will not work. However, search engines are much more likely to look for gold, platinum, copper, silver, etc. in old abandoned rural estates...
With any movement of the device, a similar action occurs with the electric field, while the magnetic field remains constant in coordinates. Therefore, the resulting movement of the spring will also be different. Where it turns out to be most intense, its source is almost certainly located - the magnetic field. Another thing is that this kind of search magnet for non-ferrous metals will not be able to show which metal is hidden under the thickness of wood or earth. But the device will definitely show that there is metal there.
Any metal can be detected by a magnetic field
The principle of operation of such a pseudo-magnet is similar to the coils of a metal detector, with the only difference being that the “magnet” will be tuned to only 1 metal and this is in theory - but we don’t know how it will behave in practice, BUT, most likely, it’s cheaper, faster and simpler will use an ordinary metal detector to search for non-ferrous metals, since not a single wizard has yet invented a magnet for non-ferrous and precious metals, maybe because there are no wizards!
Numerical experiment
And now there will be something for which all this was started.
They brought up a theory here, you know. What is she capable of? What if it’s just like a shadow on a fence? Or it doesn’t work at all... First you need to understand the geometry of the problem. Our video is from MIT, therefore it is American. I’ll try to guess the dimensions of their demo setup in inches (they like to measure everything in inches). The size of the magnet is like inches in diameter. This is one of those that are on sale. Then the mass of such a magnet will be approximately g. The size of the copper pipe in length is similar to inches (1 foot), and the inner and outer diameters of the pipe are most likely inches, inches.
We seem to have figured out the geometry. Now the physical properties. Copper conductivity S/m.
It was written here earlier that I could not relate the residual magnetization of a neodymium magnet to its equivalent magnetic moment. But there were kind people in the comments. User DenisHW suggested a source (see point 5 in the bibliography) where you can read it, helped you make the necessary calculations, and even checked them on the FEMM simulator.
Calculation of the magnetic field of a NdFeB ball using the FEMM simulator. Image courtesy of DenisHW
So what did we find out? NdFeB magnet belongs to the class of paramagnets, since under the influence of an external field, the internal field is enhanced. Moreover, the NdFeB alloy is able to maintain the internal field after the external field ceases. This fact classifies NdFeB as a ferromagnet. If we designate the induction of the internal field of the magnet as , and the intensity of the external magnetic field as , then the equality holds
Here is the magnetic susceptibility of the substance, and is the magnetization vector of the substance.
When a magnet is made in a factory, it is magnetized by an external field, and then the external field is turned off, with the magnet retaining some residual magnetization. It is known that for neodymium magnets the residual magnetization is approximately T. Now, if we exclude the external field from the previous equation, we get
Where do we find the magnetic moment per unit volume of the material as To find the magnetic moment of the magnet as a whole, you need to multiply by the volume of the ball For residual magnetization T you get Am². Below is a graph of the magnetic field components depending on the radial coordinate in our problem at a distance of half the diameter of the ball.
-component of the magnetic field near the surface of a permanent magnet
Once upon a time it was possible to measure with a device. The fields directly on the surface of such magnets are usually less than the residual magnetization and are on the order of several thousand gauss. What I measured for the rectangular magnet was about 4500 Gauss. Therefore, we have a completely realistic result on the magnetic field graph.
Now let's use the solution to the equation of motion to plot the speed of the magnet. For all the parameters chosen above, the coefficient of friction is equal to N/(m/s), the steady-state speed is cm/s - just about 3 inches per second! In the video, the ball passes through a 12-inch long pipe in about 4 seconds.
Graph of the solution to the equation of motion of a magnet in a copper pipe
THIS IS AWESOME!
I know that it is correct to write “test” through “e”, but in this case it would be more correct through “o”.
And we continue. The dissipated power turns out to be approximately mW, and the characteristic time to reach a steady state is ms. Below are graphs for two different initial speeds: zero, and cm/s.
And in addition, user vashu1 rightly noted that it would be nice to know the current induced in the copper tube. Well, this is possible too. Let's integrate
It is necessary to integrate over exactly the semi-infinite limits, since in the other half of the pipe the current flows in the opposite direction. My answer turned out to be A. To be honest, I didn’t expect such a large current to be generated. The user vashu1 got 50 A, which, apparently, is also not far from reality. I think vashu1 calculated the sum of the currents in the entire pipe, which is also reasonable for power reasons.
This is how the research turned out. I hope it was interesting. Leave your comments. I will try to answer everyone. If you liked the article, support the author with a like or a plus in karma. Thanks for reading.
How to assemble and set up
It will be very difficult to find/buy a rhenium spring, but all other parts of the device are quite accessible for making yourself. The sequence is:
- A steel axle is made from a thin-walled steel pipe with a diameter of no more than 16 mm. Its length should not be less than three diameters, otherwise the change in the magnetic field cannot be detected.
- A frame is made from thin copper or brass wire. The authors do not describe its dimensions, but, based on the dimensions of the tubular axis, it should be at least 200x200 mm. The frame must be sufficiently rigid.
- Three (as many as possible) holes are drilled in the tubular axle at equal distances, in which the wooden axles are placed.
- Thin-walled wooden disks are made, the number of which must correspond to the number of holes drilled in the axle. Obviously, discs can also be made of plywood: what matters is the mass of the disc and its absolute immunity to magnetic fields.
- The central sectors of each disk are covered with metal foil made of the metal that will be searched. Thus, a search magnet for non-ferrous metals - copper, gold and silver (platinum is searched for much less frequently) should have three sets of replaceable wooden disks.
- The frame with disks must be able to move freely along the entire tubular axis with fixation in a certain place. If the fits of the mating parts are made with the required accuracy, then there should be no swaying of the frame when it moves.
- To create a magnetic trap, plates from an old transformer are used, which are packed into the frame outline. The distance between adjacent plates should not exceed 1.5 mm in thickness, and 5...6 mm in length. Such plates form the screen of the device that perceives magnetic radiation.
- Next, assemble the magnetic coil. You will need a solenoid made of 600 layers of enameled wire, which is connected to an alternating current voltage source. The winding should be multilayer, this will reduce the parasitic capacitance of the coil and make the device less inertial.
- A ferromagnetic or - which is better - a ferroelectric core is inserted inside the coil.
- By connecting this structure through a step-down transformer, a constant position of the frame with the plates is achieved relative to the wooden disks. This will be the conditional zero of the search “magnet” for non-ferrous metals.