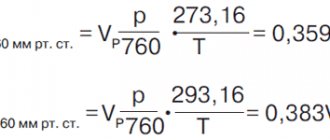Calculation of specific gravity of water
Liquids.
In nature, there are four types of states of matter: solid, liquid, gaseous and plasma. The main difference between liquids and solids is their fluidity, i.e. the ability to easily take the shape of the vessel in which the liquid is placed, while the volume of the liquid does not change. Gas also has fluidity, but at the same time it occupies any volume given to it. In vessels, liquid forms a free surface, but gas does not have a similar surface. However, from the point of view of mechanics, both liquid and gas obey the same laws if the compressibility of the gas can be neglected. Therefore, in hydraulics, the term “liquid” refers to both liquids themselves (which are often called droplet liquids) and gases (gaseous liquids).
The main properties of a liquid (when considering problems of fluid mechanics) are density, the ability to change its volume when heated (cooled) and pressure changes, and the viscosity of the liquid. Let us consider each of the properties of the liquid in more detail.
Liquid density.
The density of a liquid ρ is its mass contained in a unit volume:
where m is the mass of the liquid; W is the volume of liquid.
Since water is the most common liquid in nature, as an example of the quantitative value of a parameter that determines a particular property of a liquid, we will give the value of the parameter in question for water.
Specific gravity.
Specific gravity γ is the weight of the liquid per unit volume:
where G is the weight of the liquid in volume W.
Density and specific gravity are related by the relationship
where g is the acceleration of gravity (g=9.81 m/s 2 ).
Temperature expansion.
This property of a liquid is characterized by a change in volume with a change in temperature, which is determined by the temperature coefficient of volumetric expansion of the liquid βt:
| The Δ sign means the difference between the initial value and the final value. That is, ΔW=Wfinal-Winitial |
for water, at t=20 °С βt = 0,00015
[1/°С].
Compressibility.
This is the property of a liquid to change its volume when pressure changes, which is characterized by the volumetric compression coefficient βp:
The reciprocal of the volumetric compression ratio is called the modulus of elasticity of the liquid E and is determined by the formula:
for water E=2×10 9 Pa.
Liquid viscosity
- the property of liquids to resist shear. This property appears only when liquids move. Viscosity characterizes the degree of fluidity of a liquid. Along with easily mobile liquids (water, alcohol, air, etc.), there are very viscous liquids (glycerin, machine oils, etc.).
The viscosity of a liquid is characterized by dynamic viscosity μ.
I. Newton put forward a hypothesis about the friction force F arising between two layers of liquid at their interface with area ω, according to which the force of internal friction in the liquid does not depend on pressure, is directly proportional to the area of contact of the layers ω and the rate of change of speed in the direction perpendicular to the direction of movement layers, and depends on the type of liquid.
Let the liquid flow along a flat bottom in layers parallel to it
Due to the braking effect of the bottom, the layers of liquid will move at different speeds. Layer velocities are shown by arrows. Let us consider two layers of liquid, the centers of which are located at a distance Δу from each other. Layer A moves with speed u, and layer B with speed u + Δu.
On area ω, due to viscosity, a resistance force F arises. According to Newton’s hypothesis, this force
the proportionality coefficient μ, in this formula is the dynamic viscosity, the ratio Δu/Δy is called the velocity gradient.
Thus, dynamic viscosity is the friction force per unit area of contact of liquid layers with a velocity gradient equal to unity.
Dimension μ - Pa • s.
I. Newton’s hypothesis, presented in the formula, was experimentally confirmed and mathematically formulated in differential form
the founder of the hydraulic theory of lubrication N.P. Petrov and is currently called Newton’s law of internal friction.
In hydraulic calculations, it is often more convenient to use another quantity characterizing the viscosity of a liquid, ν:
This quantity is called kinematic viscosity. Dimension v - m 2 /s
The name “kinematic viscosity” does not have any special physical meaning, since the name was proposed because the dimension v is similar to the dimension of velocity.
The viscosity of a liquid depends on both temperature and pressure. The kinematic viscosity of droplet liquids decreases with increasing temperature, but the viscosity of gases, on the contrary, increases with increasing temperature. The kinematic viscosity of liquids at pressures encountered in most cases in practice depends little on pressure, and the viscosity of gases decreases with increasing pressure.
The viscosity of a liquid is measured using viscometers of various designs.
Fluids for which Newton's law of internal gravitation is valid are called Newtonian. There are liquids that do not obey regular formulas, these include polymer solutions, hydraulic mixtures of cement, clay, chalk, etc. Such liquids are non-Newtonian.
By the way, I have already found the formulas that plumbers and engineers need, I will describe them in other articles. Write comments, I will definitely answer your questions and try to adjust the articles to suit your needs.
Thermal conductivity of water depending on temperature at atmospheric pressure
The table shows the thermal conductivity of liquid water at normal atmospheric pressure. The thermal conductivity of water is indicated depending on the temperature in the range from 0 to 100°C.
When water is heated, it becomes more thermally conductive—its thermal conductivity coefficient increases. For example, at 10°C water has a thermal conductivity of 0.574 W/(m deg) , and as the temperature rises to 95°C, the thermal conductivity of water increases to a value of 0.682 W/(m deg).
Thermal conductivity of water depending on temperature
| t, °С | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 50 | |
| λ, W/(m deg) | 0,569 | 0,572 | 0,574 | 0,587 | 0,599 | 0,609 | 0,618 | 0,627 | 0,635 | 0,648 |
| t, °С | 55 | 60 | 65 | 70 | 75 | 80 | 85 | 90 | 95 | 100 |
| λ, W/(m deg) | 0,654 | 0,659 | 0,664 | 0,668 | 0,671 | 0,674 | 0,677 | 0,68 | 0,682 | 0,683 |
The concept of density, specific gravity and specific volume of sea water
CHAPTER 9. DENSITY OF SEA WATER
§ 33. The concept of density, specific gravity and specific volume of sea water
Specific gravity of sea water
The specific gravity of sea water is the ratio of the weight of a unit volume of sea water at temperature t to the weight of a unit volume of distilled water at the same temperature and normal atmospheric pressure.
In oceanology, the standard temperature is 17.5 ° C (the average temperature of a laboratory room), to which the value of the specific gravity of sea water measured at any temperature is reduced.
In oceanographic practice, the concept of conditional specific gravity was introduced
The specific gravity and density of sea water deviate slightly from unity, so to shorten the entry, subtract one from the number expressing the specific gravity and move the decimal point three places to the right. For example, the specific gravity ρ17.5 = 1.02624 is written as 26.24.
In oceanology, the density of sea water is understood as the specific gravity of sea water at the temperature it had in a given place, at a given depth (in situ), related to distilled water at the temperature of its highest density of 4 ° C.
For the same reason of small changes and the need for high accuracy of definitions, the concept of conditional density was introduced
When solving some hydrophysical problems, instead of Ϭt, the conditional specific gravity at 0° C (Ϭ) is used
In many hydrodynamic calculations, instead of conditional density, it is more convenient to use its inverse value, called specific volume, i.e. volume per unit mass
Since the specific volume is always greater than 0.9 and less than 1.0, then, by analogy with conventional specific gravity and density, the concept of conventional specific volume was introduced
Oceanological tables
Based on laboratory studies by the Commission of the International Council for the Exploration of the Seas (1889), the relationships between chlorine content, salinity, relative specific gravity and relative density at a temperature of 0°C were established. Empirical formulas relating these quantities were used to calculate tables published in various international manuals (for the first time in Knudsen’s tables, 1901) and in the domestic “Oceanological Tables” compiled by N. N. Zubov. In table 14 shows a sample table of correspondence of values (from the “Oceanological Tables”).
Correspondence of Cl, S, Ϭ and ρ 17.5
| Cl | S‰ | Ϭ | ρ17.5 |
| 19,00 | 34,33 | 27,58 | 26,22 |
| 19,01 | 34,34 | 27,60 | 26,23 |
| 19,02 | 34,36 | 27,61 | 26,24 |
| 19,03 | 34,38 | 27,63 | 26,26 |
Using tables, having determined the conditional specific gravity ρ17.5 by hydrometry, you can obtain the values of Cl (chlorine), S (salinity) and Ϭ (specific gravity). By determining the chlorine content by titration, the values of S‰, ρ17.5 and Ϭ0 can be obtained.
The “Oceanological Tables” provide tables for direct determination of conditional density and specific volume based on temperature and salinity.
Source: General hydrology, Gidrometeoizdat, Leningrad, 1973
Source
Interesting historical information
Initially, water in the state of melting ice was taken as the standard; over time, this rule changed, and liquid under conditions of normal atmospheric pressure and in the state of greatest density was taken as the sample.
In 1879, the International Committee of Weights and Measures equated 1 liter to 1 cubic meter. dm. From 1901 to 1964 the concept of a unit of measurement began to differ. During this period, a liter corresponded to a kilogram of water at a temperature of 3.98 ° C and a pressure of 760 mm Hg. Art. In 1901-1964. The weight of a liter of water without impurities at the established indicators was equal to 1 kg.
In 1964, at the international level, the value of the liter, which was used until 1901, was returned, and it again began to correspond to the volume of 1 cubic meter. dm, regardless of weight.
What is considered specific gravity and how to calculate it using formulas
Among the many parameters characterizing the properties of materials, there is also specific gravity. Sometimes the term density is used, but this is not entirely correct. But one way or another, these two terms have their own definitions and are used in mathematics, physics and many other sciences, including materials science.
Concept in physics
Specific gravity in physics is defined as the weight of a substance per unit volume. In the SI measurement system, this value is measured in N/m3. To understand how much 1 N/m3 is, it can be compared with the value of 0.102 kgf/m3.
To know how to calculate y. c., you need to understand the calculation formula. The formula looks like this:
where P is body weight in Newtons; V—body volume in cubic meters.
If we consider simple water as an example, we will notice that its density and specific gravity are almost the same and change very little with changes in pressure or temperature. Her y. V. equal to 1020 kgf/m3. The more salts are dissolved in this water, the greater the value of y. V. This figure for sea water is much greater than for fresh water, and is equal to 1150 - 1300 kgf/m3.
The scientist Archimedes once noticed a long time ago that a buoyant force acts on a body immersed in water. This force is equal to the amount of liquid that the body displaced. When a body weighs less than the volume of displaced fluid, it floats on the surface and goes to the bottom if the situation is the opposite.
Density of water
Water is considered a very common and frequently encountered liquid substance in everyday life. Considering the main parameters of the density and viscosity of this substance, we obtain a density under natural conditions equal to 1000 kilograms per cubic meter. This value is used in distilled water. For sea water, the density value is slightly higher and amounts to 1030 kilograms per cubic meter. This value does not seem final and is very closely related to the temperature indicator. Perfect data can be recorded at a temperature of approximately +4°C.
Specific gravity formula
The formula for calculating HC looks like the ratio of weight to volume. To calculate hydrocarbons, it is permissible to use the calculation algorithm, which is set out in a school physics course. To do this, it is necessary to use Archimedes' law, or more precisely, the definition of the force that is buoyant. That is, a load with a certain mass and at the same time it floats on the water. In other words, it is influenced by two forces - gravity and Archimedes.
The formula for calculating the Archimedean force is as follows
where g is the hydrocarbon liquid. After the substitution, the formula takes the following form: F=y×V, from here we obtain the formula for the shock load y=F/V.
Specific Gravity Calculation
“How to calculate the specific gravity of metals?” - this question often occupies those who develop heavy industry. This procedure is needed in order to find among the various variations of metals those that will have better characteristics.
The features of various alloys are as follows: depending on what metal is used, be it iron, aluminum or brass, of the same volume, the alloy will have a different mass. The density of a substance, calculated using a certain formula, is directly related to the question that workers ask when processing metals: “How to calculate specific gravity?”
As mentioned above, y. V. is the ratio of a body's weight to its volume. Do not forget that this value is also defined as the force of gravity of the volume of the substance being determined as a basis. For metals they have. V. and density are in the same ratio as the weight to the mass of the subject. Then you can use another formula that will answer the question of how to calculate the specific gravity: s.v./density = weight/mass=g, where g is a constant value. The unit of measurement is y. V. metals is also N/m3.
Thus, we have come to the conclusion that the specific gravity of a metal is called the weight per unit volume of a dense or non-porous material. To determine y. c., you need to divide the mass of dry material by its volume in an absolutely dense state - in fact, this is the formula used to determine the weight of the metal. To achieve this result, the metal is brought into such a state that there are no pores left in its particles and it has a uniform structure.
What is the relationship between density and specific gravity of a liquid?
The specific gravity of a liquid (γ) is another parameter on which its properties depend.
Specific gravity is the weight of a liquid contained in a unit V (volume).
Its value is found using the formula:
Where G is the weight of the liquid, V is the volume.
There is a direct relationship between the specific gravity and the density of the liquid medium. The formula for determining specific gravity contains the equality:
The difference between specific gravity and density is the fact that it depends on the location of the measurements, incl. on altitude above sea level and latitude.
Source
Calculate weight by age and height
table for calculating the optimal weight according to the parameters height, age
The following weight determination method is not a calculation formula. This is a ready-made table with which you can calculate the correct weight by age. And if the previous version gives an approximate norm of human body weight, then the Egorov-Levitsky table, as it is also called, displays the maximum permissible weight value, exceeding which is considered unacceptable for a given height and age group.
All you need is to know your height, age and current weight. Look for the intersection of these parameters in the table and understand how far you are from the maximum permissible value. If the number in the table is higher than your existing weight, good, if lower, there is reason to think about the gym and dietary restrictions.
Thermophysical properties of water at the saturation line (100…370°C)
The table shows the thermophysical properties of H2O water on the saturation line depending on temperature (in the range from 100 to 370°C). Each temperature value at which water is in a state of saturation corresponds to its saturated vapor pressure. At these parameters, the liquid and its vapor are in a state of saturation or thermodynamic equilibrium.
The table shows the following thermophysical properties of water in the state of a saturated liquid:
Other properties of water such as density, thermal conductivity, specific heat capacity, and thermal diffusivity tend to decrease their values as its temperature increases. For example, the density of water decreases from 958.4 to 450.5 kg/m 3 when heated from 100 to 370°C.
The thermal conductivity of water in a state of saturation also decreases with increasing temperature (in contrast to normal conditions and temperatures up to 100°C, at which it increases during the heating process). The decrease in thermal conductivity is associated with an increase in both the temperature and pressure of the saturated liquid.
It should be noted that the specific enthalpy of water , depending on temperature, increases significantly when heated, both to the boiling point and above.
Determination of specific gravity
The physical quantity, which is the ratio of the weight of a material to the volume it occupies, is called the HC of the material.
Materials science of the 21st century has gone far ahead and technologies that were considered science fiction a hundred years ago have already been mastered. This science can offer modern industry alloys that differ from each other in qualitative parameters, but also in physical and technical properties.
To determine how a certain alloy can be used for production, it is advisable to determine the HC. All objects made with the same volume, but different types of metals were used for their production, will have different masses, it is in a clear connection with volume. That is, the ratio of volume to mass is a certain constant number characteristic of this alloy.
To calculate the density of a material, a special formula is used, which has a direct connection with the HC of the material.
By the way, the HC of cast iron, the main material for creating steel alloys, can be determined by the weight of 1 cm3, reflected in grams. The more HC the metal, the heavier the finished product will be.
Calculate weight using BMI (Quetelet body mass index)
table for calculating optimal weight using Quetelet body mass index
Using the Body Mass Index, you can find out in what predetermined range a person’s current weight is: deficit, normal or obese (all BMI values are shown in the table).
BMI is calculated using a formula that takes into account the initial values of height in meters and weight in kilograms. The formula looks like this: KMT = weight in kilograms: (height in meters * height in meters).
Example: a man with a height of 185 cm (1.85 m) and a weight of 88 kg will have a BMI = 88: (1.85 * 1.85) = 27.7. We look for the value in the table and understand that the index is in the range of Overweight (pre-obesity).
An important point: calculating the correct weight according to BMI does not take into account gender and age-related changes in the body.
Calculation of the weight of other liquids
To calculate the weight of a liquid substance, you need to know its density. The table shows the weight of 1 liter of the most common liquids in everyday life:
| Liquid name | Weight 1 l, kg |
| Wine | 0,97-0,99 |
| Kefir | 1,02-1,04 |
| Juice | 1,05 |
| Nectar | 1,0 |
| Alcohol | 0,79 |
| Sunflower oil | 0,92-0,93 |
| Honey | 1,40-1,44 |
Specific gravity in medicine
Specific gravity in medicine is a fairly common concept. It is used for analysis. It has long been known that u.v. water is proportional to the concentration of dissolved substances in it; the more of them, the greater the specific gravity. U.v. distilled water at 4 degrees Celsius is 1.000. It follows that u.v. urine can give an idea of the amount of substances dissolved in it. From here you can make one or another diagnosis.
The specific gravity of human urine ranges from 1.001 to 1.060. Young children have less concentrated urine with readings ranging from 1.002 to 1.030. In the first days after birth, the specific gravity of urine ranges from 1.002 to 1.020. According to these data, doctors can judge the functioning of the kidneys and make one or another diagnosis.
Among the many parameters characterizing the properties of materials, there is also specific gravity. Sometimes the term density is used, but this is not entirely correct. But one way or another, these two terms have their own definitions and are used in mathematics, physics and many other sciences, including materials science.
Specific gravity
Liquid density
Each liquid substance has its own individual qualities and parameters. In physics, a certain number of phenomena associated with these original parameters are traditionally considered.
Liquid substances are usually divided into two main parts:
These categories of liquid substances have significant differences among themselves. Droplet liquid substances are significantly different from gaseous substances. These substances have a specific volume. The size of this volume does not change under the influence of certain external forces. In the gas state, liquid substances are completely distributed throughout the entire volume in which they are located. In this case, a similar category of liquid substances will reduce or increase its actual volume to a large extent, under the influence of external forces and depending on their magnitude. Liquid substances of each type have three properties that these substances cannot lose:
These parameters have the ability to influence multiple laws of their movement; for this reason, these properties are basic at the stage of their research and use of information for practical purposes.
Metals with the highest and lowest specific gravity
In addition to the concept of specific gravity used in mathematics and physics, there are also quite interesting facts, for example, about the specific gravities of metals from the periodic table. If we talk about non-ferrous metals, then the heaviest ones include gold and platinum.
These materials exceed in specific gravity such metals as silver, lead and many others. “Light” materials include magnesium with a weight lower than that of vanadium. We must not forget about radioactive materials, for example, the weight of uranium is 19.05 grams per cubic cm. That is, 1 cubic meter weighs 19 tons.
Specific gravity of other materials
It is difficult to imagine our world without many materials used in production and everyday life. For example, without iron and its compounds (steel alloys). The HC of these materials fluctuates in the range of one to two units and these are not the best results. Aluminum, for example, has low density and low specific gravity. These indicators allowed it to be used in the aviation and space industries.
Specific gravity of metals
Copper and its alloys have a specific gravity comparable to lead. But its compounds - brass and bronze are lighter than other materials, due to the fact that they use substances with a lower specific gravity.
Source
Thermal conductivity of water depending on temperature and pressure
The table shows the thermal conductivity values of water and water vapor at temperatures from 0 to 700°C and pressure from 1 to 500 atm.
As you know, water boils at atmospheric pressure and turns into steam at a temperature of 100°C. The thermal conductivity coefficient of water under these conditions is 0.683 W/(m deg). As pressure increases, the boiling point of water also increases (Clapeyron-Clausius law). According to the table, it can be seen that at a pressure 100 times higher than atmospheric pressure (100 bar), water is in the form of steam at a temperature of 310°C and has a thermal conductivity of 0.523 W/(m deg).
Thus, it should be noted that changes in pressure affect both the boiling point of water and the value of its thermal conductivity . High thermal conductivity of water is achieved due to increasing pressure - with increasing pressure, the thermal conductivity coefficient of water increases. For example, at a pressure of 1 bar and a temperature of 20°C, water has a thermal conductivity of 0.603 W/(m deg). When the pressure increases to 500 bar, the thermal conductivity of water becomes equal to 0.64 W/(m deg) at the same temperature.
Source