29Jan
- By: Semantics
- Uncategorized
- Comments: 0
When manufacturing metal, the enterprise uses a classification of workpieces according to structural features.
Metallurgists usually observe changes in structure during metalworking, including after heat treatment. And one of these states is austenite, and after quenching followed by cooling, pearlite, martensite and other changes can be obtained. In this article we will talk about which steels belong to the austenitic class and what properties these materials have. This formation can be obtained in a steel billet, that is, in a solution of iron with the addition of carbon. The peculiarity of this state lies in the arrangement of the atoms of these substances. They sequentially form a pattern in one of two options:
- BCC A-Fe. This is a body-centered structure, according to which the atoms are arranged like this: they are at each vertex of the cube (there are 8 in total), and there is also one in the very center). This option does not happen often, on average in 10% of cases.
- FCC U-Fe. The volume of the structure is preserved, but the same number is added to the previous vertex points - they are placed in the center of each face. But there is no atom in the core. Thus, there are 16 of them in total. This is the most frequently appearing structure - face-centered. It is very strong in relation to low and high temperatures, as well as loads.
If we say what “austenitic steel” means simply, then it is a special structure of the metal that determines the technical characteristics of the alloy. When its state changes (heating, cooling, etc.), its properties also change. It is thanks to passing through austenite with subsequent cooling that such popular heat treatment as hardening is possible (heating above the critical point - until the crystal lattice changes). This procedure is popular because it is an excellent, inexpensive and fairly technologically simple way to increase the strength of metal.
This modification of the metal is characterized by a high degree of alloying (the most common alloying additive is chromium). Its peculiarity is the presence of a face-centered lattice, and the fact that it is preserved even in extreme cold. The main characteristics of austenites are strength and resistance to deformation even when heated. All this allows the use of products made from the material in the most dangerous, aggressive environments; they are very actively used in mechanical engineering, as well as in the chemical and oil industries.
Steel group 300
Chromium-nickel stainless steels, depending on the internal microstructure of the structure, are divided into austenitic, austenitic-martensitic and austenitic-ferritic.
The structure of these steels depends on the content of carbon, chromium, nickel and other elements. Such steels are used in mechanical engineering, the chemical industry, the food industry, rocketry, shipbuilding, medicine and aviation. Since group 300 steels are the most commonly used steels, we will not describe in detail what everyone knows. Let us dwell only on alloying steel with titanium (Ti), which is associated with the fight against the so-called intergranular corrosion.
To reduce the tendency of steels to MCC, strong carbide-forming elements - titanium or niobium - are introduced into their composition in an amount equal to five times the carbon content. In this case, carbides such as TiC and NbC are formed, and chromium remains in solid solution. What is intergranular corrosion? Heating of steels containing a large amount of chromium in the range of 400-800°C leads to the precipitation of chromium carbide grains Cr23C6 in the boundary zones and, therefore, depletion of these zones in chromium below the 12% limit. This causes a decrease in the electrochemical potential of the boundary areas of the austenite grain and their dissolution in a corrosive environment. Corrosive destruction is intergranular in nature, leads to embrittlement of steel, and is called intergranular corrosion (ICC).
Another way to combat MCC is to produce stainless steels with minimal (less than 0.04%) carbon (C) content. In such steels (for example, AISI 304L, 316L), the formation of chromium carbides Cr23C6 is sharply limited due to the small amount of carbon.
I would also like to note that steels of group 300, contrary to general opinion, can have magnetic properties, especially after mechanical treatment and deformation, as well as during slow cooling after high-temperature heating or exposure in the temperature range from 400 to 900 degrees Celsius.
Steel grade AISI 304 Brief characteristics of steel Low carbon steel, austenitic, non-hardening, corrosion resistant, non-magnetic (if cold worked) under weak magnetization conditions. Easy to weld, resistant to intercrystalline corrosion. High strength at low temperatures. Can be polished. Steel grade AISI 321 Brief characteristics of steel Chrome-nickel steel with the addition of titanium (Ti), austenitic, non-hardening, non-magnetic, especially recommended for the manufacture of welded structures and for use at temperatures between 400°C and 800°C, resistant to corrosion.
Intergranular corrosion in austenitic stainless steels
The tendency of steel to intergranular corrosion manifests itself as a result of the precipitation of carbide phases. Therefore, when assessing the corrosion properties of steel, the most important factor is the thermokinetic parameters of the formation of carbides in it.
The susceptibility to intergranular corrosion of hardened steel type 18-10 is determined, first of all, by the concentration of carbon in the solid solution. An increase in carbon content expands the temperature range of steel's susceptibility to intergranular corrosion.
Steel type 18-10, when held in the range of 750-800 ºС, becomes prone to intergranular corrosion:
- with a carbon content of 0.084% - already within 1 minute;
- with a carbon content of 0.054% - within 10 minutes;
- at a carbon content of 0.021 5 – after more than 100 minutes.
As the carbon content decreases, the temperature simultaneously decreases, which corresponds to the minimum duration of isothermal exposure before the onset of intergranular corrosion.
What are austenitic steels
Alloy steels with 8%-10% nickel introduced into the structure acquire different properties. Nickel is capable of maintaining the austenite phase at room temperature, up to melting. In the crystal lattice of the metal, iron atoms are replaced by nickel. The shape has a cube-like structure.
This ensures a strong connection and imparts various specific properties. Such metals have corrosion resistance and good ductility. This type is used in the food industry, mechanical engineering, and oil refineries. For example, several types of steels 08Х18Н10Т, AISI 306, AISI 316.
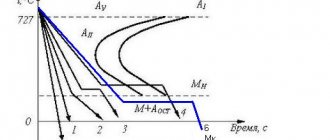
At temperatures above 570 degrees, the austenite phase decomposes into ferrite and ledeburite. In pure iron, the austenitic state is observed from 910 to 1401 degrees. In carbon steels, solid solution (austenite) exists just below 727 Celsius. When carbon replaces iron atoms. The austenitic structure can exist both in the entire crystal lattice and in the upper layers of the metal.
There are other alloys with increased resistance to corrosion at high temperatures. They are also called heat-resistant with moderate working pressure and heat-resistant with load. The operation of such steels takes place at temperatures up to 1100 degrees. Such steels include grades 08Х16Н9М2, 10Х14Н16Б, 10Х14Н14В2БР. Used in exhaust system turbines, production of intake and exhaust valves, in engine heads. Where does dynamic loading occur at high combustion temperatures?
And also cold-resistant alloys used in cryogenic installations for liquefying gases, freezing various cells, and the like. The range of operation of such steel is very large. But at room temperature its properties weaken. The main feature is corrosion resistance to liquid nitrogen and other substances. There are several types of steels with these properties: 03Х20Н16AG6, 7Х13Н4AG2. All known steels adhere to the standards in accordance with GOST 5632-72.
All steels with an austenitic lattice structure belong to the class of corrosion-resistant at various operating temperatures over a wide range. Such steels are difficult to machine. Poor thermal conductivity makes hot forging difficult to use. And not all stainless steel can be hardened. Leads to loss of its properties. Most metals have good toughness. The cutting part of the tool is subject to corrosion diffusion. Material sticking to the tip of the cutter. With slight deformation, the material itself becomes compacted, which leads to a change in physical properties. This justifies the costs of producing such steels and its value.
- Forge welding of Damascus steel Even though forge welding has long given way to arc welding in the industry, there are still areas in which it is still in demand. In particular, knife makers use it...
- Niat-5 electrodes Welding electrodes based on nickel, chromium, molybdenum and nitrogen. Important components of the rod. Restrictions on welding in ceiling mode and downhill. When nitrogen concentrations occur in the weld metal, pores form. Brand...
- Welding electrodes LF-2 Electrodes for cast iron LF-2 speak for themselves. Surfacing on cast iron of the second type. Suitable for malleable graphite and high-strength cast iron. Mainly to eliminate casting crack defects. The electrode wire contains...
- Electrodes TsL-9 Universal electrodes for welding stainless steel and dissimilar steels are represented by the TsL-9 brand. The first purpose is for welding two-layer steel from the side of the surface exposed to aggressive environments. In other words, the seam withstands the impact...
- Technology of welding aluminum with steel A reliable way to weld iron and aluminum through bimetal. Bimetal is a composite material consisting of several layers of dissimilar metals. Methods for its manufacture by simultaneous rolling through shafts. Diffusion of molecules occurs between the layers...
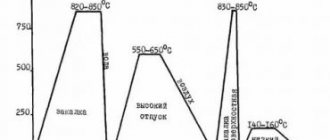
Heat Treatment Properties
Heat-resistant and heat-resistant grades can be subjected to different types of heat treatments to increase beneficial properties and modify the existing grain structure. We are talking about the number and principle of distribution of dispersed phases, the size of blocks and grains themselves, and the like.
Annealing such steel helps reduce the hardness of the alloy (sometimes this is important during operation), as well as eliminate excessive brittleness. During the processing process, the metal is heated to 1200 degrees for 30-150 minutes, then it must be cooled as quickly as possible. Alloys with a significant amount of alloying elements are usually cooled in oils or in open air, while simpler alloys are cooled in ordinary water.
Double hardening is often carried out. First, the first normalization of the compositions is performed at a temperature of 1200 degrees, followed by a second normalization at 1100 degrees, which allows for a significant increase in plastic and heat-resistant properties.
Increased heat resistance and mechanical strength can be achieved through the process of double heat treatment (hardening and aging). Before operation, artificial aging of all heat-resistant alloys is carried out (that is, they are dispersion hardened).
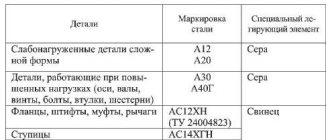
Decoding stainless steel grades
In order to choose the right grade of corrosion-resistant steel for certain purposes, it is most convenient to use special reference books. They provide information about all possible designation options for such alloys in different countries of the world. Among the huge variety of brands, we can highlight those that are most widespread among specialists in many countries around the world. These include the following grades of stainless steels with an austenitic structure.
- 10Х13Н17М3Т, 10Х13Н17М2Т: these grades are distinguished by, in addition to exceptional corrosion and thermal resistance, a good ability to form welded joints. Thanks to these qualities, products made from alloys of these brands can be successfully operated at elevated temperatures and come into contact even with very aggressive environments. The constituent elements of such alloys, which determine their unique characteristics, are: chromium (16-18%), molybdenum (2-3%), nickel (12-14%), carbon (0.1%), silicon (0. 8%), copper (0.3%), titanium (0.7%), manganese (2%), sulfur (0.02%), phosphorus (0.035%). In other countries, these brands are designated differently, in particular: in China - OCr18Ni12Mo2Ti, in Japan - SUS316Ti, in the USA - 316Ti, in France - Z6CNDT17-12.
- 08Х18Н10, 08Х18Н9: these steel grades are used for the production of pipes of various sections, elements of furnace equipment, and at chemical industry enterprises. The composition of such steels includes: chromium (17-19%), titanium (0.5%), nickel (8-10%), carbon (0.8%).
Stainless steel ducts
- 10Х23Н18: stainless steels of this grade belong to the heat-resistant category. When using them, be aware that they may become brittle when tempered. The composition of steels of this grade includes: chromium (22-25%), nickel (17-20%), manganese (2%), silicon (1%).
- 08Х18Н10Т: stainless steel products of this brand are welded well even without preheating and do not lose their corrosion resistance even at high temperatures. The insufficiently high strength that steels of this grade are characterized by is easily improved by heat treatment, which is recommended by GOST 5632-72.
- 06ХН28МДТ: a unique grade of steel, welded structures from which can be successfully operated even in very aggressive environments. The composition of this grade of corrosion-resistant steel includes: chromium (22-25%), nickel (26-29%), copper (2.5-3.5%).
- 12Х18Н10Т: products made from this grade of steel, characterized by high thermal stability and exceptional impact toughness, are mainly used in oil refining enterprises, in the chemical, pulp and paper industries, as well as in construction.
Correspondence table of the main grades of stainless steels and chemical composition
Stainless steel grades with a martensitic structure include: 40Х13, 20Х13, 12Х13, 30Х13. Products made from these grades of stainless steel cannot be joined by welding; they are mainly used to make cutting and measuring tools and spring elements. The great advantages of such products are the almost complete absence of internal defects (flocks) in them; moreover, they do not become more fragile after tempering.
Corrosion-resistant steels with a ferritic structure include: 08Х17, 08Х18Т1, 08Х13. It is not recommended to manufacture parts from these grades of steel that will experience significant shock loads and operate at low temperatures.
In order to understand the qualitative and quantitative composition of stainless steel, it is enough to decipher its brand. The algorithm for this decryption is quite simple:
- using the first number in the steel grade, the quantitative content of the main element after iron is determined - carbon (calculated in hundredths of a percent);
- The content of other elements in the steel (calculated in whole percentages) is determined by the numbers behind the letters by which such elements are designated (X - chromium, H - nickel, M - molybdenum, etc.).
A wide range of stainless steel grades allows you to find the best option for yourself. It should be borne in mind that certain types of stainless steel can be interchanged within certain limits. If you encounter difficulties when choosing steel, you need to contact technical consultants of specialized companies.
Stabilizing annealing of austenitic chromium-nickel steels
In unstabilized steels, annealing is carried out in the temperature range between the heating temperature for hardening and the maximum temperature for the manifestation of intergranular corrosion. The value of this interval primarily depends on the chromium content in the steel and increases with increasing its concentration.
In stabilized steels, annealing is carried out to transfer carbon from chromium carbides to special titanium and niobium carbides. In this case, the released chromium goes to increase the corrosion resistance of steel. The annealing temperature is usually 850-950 ºС.
Strength - austenitic steel - Great Encyclopedia of Oil and Gas, article, page 1
Strength – austenitic steel
The strength of austenitic steels generally increases with increasing chromium, nickel and carbon content.
Under the influence of cold hardening, the strength of austenitic steels can increase by more than 2 times, hardness by 2–5–3 times, while ductility decreases by more than 4 times, and impact toughness by 7 times. Austenitic steel becomes magnetic after hardening, as part of the austenite turns into ferrite. The greater the degree of deformation, the stronger the magnetic properties appear. These properties of stainless steels create certain difficulties during cold machining (cutting, bending), for example: cutting tools for processing stainless steels must be well sharpened, special finishing of the cutting edges is desirable; when working with a blunt tool, a hardened surface is formed, which complicates further processing; cutting is usually carried out with abundant cooling with emulsions.
One of the ways to increase the strength of austenitic steels for cryogenic technology is to alloy them with nitrogen, which, like carbon, forms interstitial solid solutions. The presence of chromium and especially manganese increases the solubility of nitrogen in steel.
The strength of heat-strengthening aluminum alloys approaches the strength of austenitic steels and therefore in many cases they could be their substitutes. Their disadvantage is their tendency to undergo stress corrosion. In addition, these alloys are softened in the weld zone.
It should be borne in mind that the strength of austenitic steels is significantly lower than the strength of high-quality structural steels.
Aging strengthening is one of the effective ways to increase the strength of austenitic steels based on Fe-Mri, Fe-Mn-Cr and Fe-Mn-Cr-Ni without losing their non-magneticity. Currently, a large number of compositions of aging steels have been developed, in which carbides, nitrides or intermetallic compounds of vanadium, tungsten, molybdenum, niobium, titanium, tantalum, zirconium, and aluminum are used as strengthening phases.
In Fig. Figure 4 shows schematic generalized curves of changes in strength (at, oh) and ductility (b, h)) of austenitic and pearlitic steels depending on the test temperature. With its increase, the strength of austenitic steels gradually decreases, most intensely in the temperature range above 600 C, and pearlitic steels - starting from 350 - 400 C. For the latter, in the temperature range 150 - 350 C, where there is a local increase in strength due to the manifestation of the effect of strain aging, deviation from this dependence.
C, a further increase in temperature reduces the degree of the process. The development of intergranular sulfide corrosion is undoubtedly facilitated by the presence of tensile stresses and the effect of adsorption reduction in the strength of austenitic steel.
Let us note in this regard that with an increase in the strain rate during stretching of hardened samples, as a result of an increase in the temperature of the metal of the working part of the sample, the tensile strength of the steel may decrease. Increasing the test temperature to 100 - 200 C reduces the tensile strength of hardened transition class steel to a level close to the strength of austenitic steel.
Cold hardening, accompanied by distortions of the crystal lattice, can very significantly increase the tensile strength and especially the yield strength, which is important in the production of wire and spring tape. The tensile strength of high carbon steel wire can be increased to 400 kg/mm2 and above by small partial reductions
Cold clasp significantly increases the strength of austenitic steels. The widespread use of cold hardening is hampered by the difficulty of its implementation on parts of complex shapes. In addition, during cold drawing and cold rolling, ductility decreases and anisotropy of properties along and across the rolled product is created. All-round compression with high specific pressures increases the mechanical properties, compacting the metal, filling atomic gaps, and slightly changing the dimensions of the crystal lattice.
Pages: 1
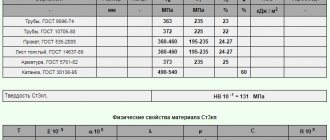
Strength characteristics of stainless steel
Products made of stainless steel, similar to products made of carbon steel, are characterized by their definition through the so-called strength classes and hardness classes. Strength regulation for stainless steel fasteners is carried out in accordance with the international standard norm ISO 3506 (GOST R ISO 3506 in the Russian Federation).
Strength characteristics of stainless bolts, screws and studs
| steel grade | Strength class | Proof of yield, MPa | Tensile strength, MPa |
| from A1 to A5 | 50 | 210 | 500 |
| 70 | 450 | 700 | |
| 80 | 600 | 800 | |
| 60 | 410 | 600 |
Markings applied to products allow you to quickly determine the material and strength class.
Strength characteristics of stainless steel nuts
| steel grade | Strength class | Test load, MPa | ||
| Nuts | Low nuts | Nuts | Low nuts | |
| from A1 to A5 | 50 | 025 | 500 | 250 |
| 70 | 035 | 700 | 350 | |
| 80 | 040 | 800 | 400 | |
The low nut category includes nut models with heights ranging from 0.5D to 0.8D (with the first value included in the range and the second not), where D is the thread diameter of the nut. Regular nuts are defined as nuts with a height of 0.8D (inclusive).
In the tabular data, the test load is defined as a safe load, the removal of which does not lead to the formation of residual deformation at the nut. Low nuts have a lower value of this parameter compared to regular nuts.
The production of markings for the strength class and grade of stainless steel is carried out by analogy with bolts made of stainless materials. We can also talk about marking the materials of nuts in an alternative version, which involves making a cut (grooves) on the edges located on the sides. A2 is one row of grooves, A4 is two rows.
Strength characteristics of stainless steel set screws
In the indications of the strength characteristics of mounting screws made of stainless steel, the concept of strength class cannot be used. The main mechanical parameter is the hardness class. It is not necessary to mark the set screws, due to the lack of the necessary surface area for marking in the most common cases. Recognizing the brand and hardness class without the required documentation will be an extremely difficult task.
| Hardness scale | Hardness class | |
| 12H | 21H | |
| Units of hardness | ||
| Vickers HV | from 125 to 209 | not less than 210 |
| According to Brinell NV | from 123 to 213 | at least 214 |
| According to Rockwell HRB | from 70 to 95 | no less than 96 |
Application of alloys
Austenitic steels are used in the manufacture of devices operating at high temperatures, starting from 200 °C: steam generators, rotors, turbines and welding mechanisms. The disadvantage of using austenite in these mechanisms is the low strength of the metal. With prolonged contact of iron alloys with various hydroxides, additional cracks may form, which will lead to breakage of the working surfaces of devices. This drawback can be eliminated by adding additional chemical elements to the iron solution: vanadium and niobium. They form a carbide phase, which increases the strength of steel.
Stainless austenitic steels are used in mechanisms operating in difficult conditions and with strong temperature changes. They are most often used when welding corrosion-resistant pipes. During this process, a seam space is created between the fasteners. When stainless austenite pipes are heated to the melting temperature, they acquire a monolithic structure that protects the metal from oxidation processes and high temperature changes.
Austenitic steels are also highly resistant to electromagnetic radiation. Therefore, it is used in the production of individual parts for electronic equipment. Austenite improves the strength of radio mechanisms and does not lose its properties when the structure of the magnetic field changes. For this reason, radio equipment will easily receive the necessary signals.
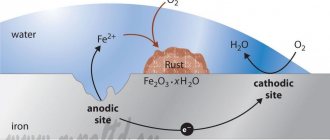
Austenitic iron alloys are widely used in the production of mechanisms operating in aqueous environments. Stainless steel is resistant to corrosion. It is used as a protective material. With the correct ratio of chromium and nickel, austenite can form a thin layer, reducing the influence of the aqueous environment on the working surface of the metal device. As a result, wear on the device is reduced. But with significant leaching of nickel, the material completely loses its resistance to corrosion.
Modern turbine casings also use high-yield austenitic steels. They allow you to avoid warping of this device and improve its strength. Due to its coarse grain structure, high yield strength austenite can also be used to strengthen the turbine rotor structure. The disadvantage of this technology is a significant increase in the cost of mechanisms due to the use of a large amount of expensive austenitic steel.
Checking stainless fasteners using a magnet
A characteristic of products made of austenitic steel may be their magnetic property. The attraction of a magnet to products A1-A5 indicates the low quality of the material. This is also defined by the international standard ISO 3506 (GOST R ISO 3506 in the Russian Federation). According to this standard, all fasteners using austenitic stainless steels are non-magnetic under normal conditions, but cold working or other mechanical processing may result in certain magnetic characteristics. The property of each material is the ability to magnetize, which can also be used for stainless steels. As is known, only vacuum is considered completely non-magnetic.
for heat-resistant chromium steels
12XM, 12MX, 15XM, 15X5M, 15X5M-U:
- At design temperatures below 20 °C, the permissible stresses are taken to be the same as at 20 °C, subject to the permissible use of the material at a given temperature.
- For intermediate design wall temperatures, the permissible stress is determined by linear interpolation with rounding the results down to 0.5 MPa.
- The permissible stresses located below the horizontal line are valid for a service life of 105 hours. For a design service life of up to 2 * 105 hours, the permissible stress located below the horizontal line is multiplied by a factor of 0.85.
Hardening of austenitic chromium-nickel steels
In steels without titanium and niobium additives, hardening means heating above the dissolution temperature of chromium carbides and fairly rapid cooling, which fixes a homogeneous gamma solution. The heating temperature for quenching increases with increasing carbon content. Therefore, low-carbon steels are hardened at lower temperatures than high-carbon steels. In general, the heating temperature range is from 900 to 1100 ºС.
The duration of exposure of steel at the hardening temperature is quite short. For example, for sheet material, the total heating and holding time when heated to 1000-1050 ºС is usually selected at the rate of 1-3 minutes per 1 mm of thickness.
Cooling from the quenching temperature must be rapid. For unstabilized steels with a carbon content of more than 0.03%, cooling in water is used. Steels with a lower carbon content and small cross-sections of the product are cooled in air.
Rules for marking corrosion-resistant steels
The designation consists of numbers and letters. The two-digit number at the beginning of the marking is the amount of carbon in hundredths of a percent. The following are letters characterizing certain alloying elements. After them are placed numbers equal to the percentage of alloying elements, rounded to the nearest whole number. If the percentage of the additive is in the range of 1-1.5, then the number is not placed after the letter. To symbolize alloying components in Russian regulatory documentation, the Russian alphabet is used:
- X – chromium;
- N – nickel;
- T – titanium;
- B – tungsten;
- G – manganese;
- D – copper;
- M – molybdenum.
Resistance of austenitic chromium-nickel steels to acids
The ability to passivate provides chromium-nickel austenitic steels with fairly high resistance to nitric acid. Steels 12Х18Н10Т, 12Х18Н12Б and 02Х18Н11 have the first resistance rating:
- in 65% nitric acid at temperatures up to 85 ºС;
- in 80% nitric acid at temperatures up to 65 ºС;
- 100% sulfuric acid at temperatures up to 65 ºС;
- in mixtures of nitric and sulfuric acids: (25% + 70%) and 10% + 60%) at temperatures up to 70 ºС;
- in 40% phosphoric acid at 100 ºС.
Austenitic chromium-nickel steels also have high resistance to solutions of organic acids - acetic, citric and formic, as well as alkalis KOH and NaOH.
Products made of ausnitic steels
Semi-finished products in which steel is supplied are:
- Sheets 4-50 mm thick with guaranteed chemical composition and mechanical properties.
- Forgings. Due to the complex processing of these steels by welding, the production of some parts involves the production of almost finished products already at the casting stage. These are rotors, disks, turbines, engine pipes.
Austenite joining methods:
- Solder greatly limits the use of metal at temperatures above 250 °C;
- Welding is possible in a protective atmosphere (gas, flux), with subsequent heat treatment.
- Mechanical connection - bolts and other fasteners made of similar material.
Austenitic steels are one of the most expensive technical steels, the use of which is limited to a narrow specialization of equipment.
Above a certain content of manganese, nickel or some other elements, the γ-state exists as a stable state from room temperature to the melting point. Such highly alloyed iron alloys are called austenitic steels. Unlike other iron alloys, austenitic steels (and ferritic) do not undergo transformations upon heating and cooling [1]. Therefore, heat treatment is not used to strengthen austenitic steels.
Those. the austenite structure is obtained with a high content of an alloying element in the steel, expanding the region of the γ-phase (Ni, Mn, etc.), in this case the steel is called austenitic or austenitic class steel
[2].
In austenitic steels, chromium provides heat resistance and corrosion resistance, nickel stabilizes the austenitic structure and increases heat resistance, ductility and manufacturability, including at high and low temperatures, which explains the widespread use of austenitic steels as structural materials for a variety of conditions (aggressive environments, high temperatures, etc.).
Heat-resistant and heat-resistant austenitic steels
Austenitic steels with fcc lattice have significantly higher heat resistance compared to steels with bcc lattice.
Heat-resistant austenitic steels
, used for the manufacture of furnace equipment parts, are characterized not only by high heat resistance (scale resistance), but also by high heat resistance.
Heat-resistant austenitic steels
include 20Х23Н18, 20Х25Н20С2, which have scale resistance up to 1100°C.
Heat-resistant austenitic steels
.
Rotors, disks, blades of gas turbines, and diesel engine valves operating at temperatures of 600-700°C are made from heat-resistant austenitic steels. Chromium-nickel austenitic steels are additionally alloyed with tungsten, molybdenum, vanadium, niobium, boron and other elements to increase heat resistance. Heat-resistant steels of the austenitic class
include steels 09Х14Н16Б, 09Х14Н19В2БР, 45Х14Н14В2М.
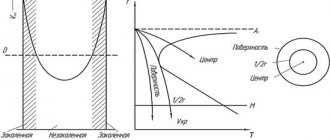
Methods for obtaining austenite
Austenite is a metal structure that occurs in low-alloy grades in the temperature range of 550-743 ºC. How can this structure and, accordingly, properties be preserved beyond the boundaries of these t? — Answer: by alloying method. When the austenite lattice is filled with atoms of other elements, structural distortions are formed, and the process of restoration of the bcc lattice (natural structure at normal temperatures) shifts by hundreds of degrees.
How these properties manifest themselves and in what state depends on the additional, i.e., alloying elements and the heat treatment of the part, which it can additionally receive. Moreover, it is not only the elements that influence, but their ratio, so austenitic steel is divided into:
- chromium-manganese and chromium-nickel-manganese (07Х21Г7АН5, 10Х14АГ15, 10Х14Г14H4T);
- chromium-nickel (08Х18Н12Б, 03Х18Н11, 08X18H10T, 06X18Н11, 12X18H10T, 08X18H10;
- high-silicon (02Х8Н22С6, 15Х18Н12C4Т10);
- chromium-nickel-molybdenum (03Х21Н21М4ГБ, 08Х17Н15М3Т, 08Х17Н13M2T, 03X16H15M3, 10Х17Н13М3Т).
Chemical elements and their effect on austenite
Austenite has few accomplices; they can be used both jointly and partially, depending on what properties need to be obtained:
- Chromium - when its content is more than 13%, it forms an oxide film on the surface, 2-3 atoms thick, which prevents corrosion. In austenite, chromium is in a free state, subject to a minimum carbon content, since it immediately forms Cr23C6 carbide, which leads to chromium segregation and depletes large areas of the matrix, making it available for oxidation; Cr23C6 carbide itself promotes intergranular corrosion of austenite.
- Carbon (its maximum value is not more than 10%). Carbon in austenite is in a combined state, its main task is the formation of carbides, which have extreme strength.
- Nickel is the main element that stabilizes the desired structure. A content of 9-12% is sufficient to transfer the steel to the austenitic class. Grinds and inhibits grain growth, which ensures high plasticity;
- Nitrogen replaces carbon atoms, the presence of which in electrochemically resistant steels is reduced to 0.02%;
- Boron - already in thousandths of a percent increases plasticity in austenite, crushing its grain;
- Silicon and manganese are not listed as the main alloying elements in the labeling, but they are the main or essential alloying elements of austenite that impart strength and stabilize the structure.
- Titanium and niobium - at temperatures above 700 °C, chromium carbide decomposes and stable TiC and NiC are formed, which do not cause intergranular corrosion, but their use is not always justified in cold-resistant steels, because it increases the limit of austenite decomposition.
Heat treatment
Austenite is processed only when necessary. The main operations are high-temperature annealing (1100-1200 °C for 0.5-2.5 hours), which eliminates brittleness. Next is quenching with cooling in oil or air.
Austenitic steel alloyed with aluminum is subjected to double hardening and double normalization:
Mechanical finishing is carried out before hardening, but after annealing.