abstract “heat treatment of metal”
ABSTRACT
Topic: “Heat treatment of metals”
Content
Introduction ________________________________________________ 3
Chapter 1. Types of heat treatment of metals _______________4
1.1.Types of heat treatment of steel ______________________________5
Chapter 2. Heat treatment of non-ferrous metals ____________8
Conclusion_______________________________________________9
List of sources used _________________________________10
Introduction.
Thermal processing of materials began soon after primitive people mastered fire. First, the meat of the prey was processed and sticks were burned into coals to make drawings on the walls of the caves. Then people discovered metals, began to forge tools from native copper, and - a couple of thousand years BC. – learned to remove cold hardening by annealing. The era of heat treatment of metals began, changing the course of history. The cheese-making process that soon appeared not only gave people iron that was stronger than copper - it marked the beginning of the chemical-thermal processing of metals. of thermomechanical processing of alloys, giving them new properties through a combination of plastic deformation and heat treatment, dates back only a century The youngest direction - thermomagnetic processing - allows you to vary the magnetic properties of special alloys.
Chapter 1. Types of heat treatment of metal.
Thermal (or heat) treatment
is a set of operations of heating, holding and cooling hard metals and alloys in order to obtain specified properties by changing the internal structure and structure. Heat treatment is used either as an intermediate operation to improve workability by pressure or cutting, or as the final operation of a technological process, ensuring a given level of product properties. The total duration of heating of the metal during heat treatment is the sum of the time of its own heating to a given temperature and the holding time at this temperature.
Among the main types of heat treatment, it should be noted: annealing, hardening, tempering, normalization.
Chemical thermal treatment (CHT) of steel is a set of heat treatment operations involving saturation of the surface of the product with various elements (carbon, nitrogen, aluminum, silicon, chromium, etc.) at high temperatures. Surface saturation of steel with metals (chromium, aluminum, silicon, etc.), which form substitutional solid solutions with iron, is more energy-intensive and longer lasting than saturation with nitrogen and carbon, which form interstitial solid solutions with iron. Chemical-thermal treatment increases hardness, wear resistance, corrosion resistance, increases reliability and durability.
Hello student
Surface carburization of steel products. Surface carburization is the process of enriching the surface of a product with carbon. Products are cemented in order to give their surface greater hardness than the internal layers.
Products intended to operate under variable shock loads and subject to abrasion are subjected to cementation. Having a hard surface with a relatively soft and viscous material of the inner layers, such products wear out little and at the same time are not breakable. So, for example, the teeth of automobile gears, made of soft material, would quickly lose the necessary precision of outline, and those made of hardened steel could break under shocks; being made of a material with a cemented surface, they fully satisfy the requirements for their work.
The thickness of the cemented layer usually ranges from 0.1 to 2.0 mm; greater and lesser depths of cementation can occur only in special cases.
Products subjected to cementation must be made of low-carbon material (not higher than 0.23% C). If the carbon content in the starting material is higher, it will not be possible to obtain a soft core after hardening the cemented product.
The carbon content in the cemented layer is usually no higher than 0.83%, since with a higher C content free cementite appears, which introduces greater fragility into the cemented layer. Greater carburization is allowed only for products that work without shocks and require very high hardness.
The carburizing operation involves bringing the carburized surface into contact with a carbon-rich substance and heating it to a temperature at which carbon can penetrate the steel fairly quickly.
The lower heating limit for carburization is point A1 (723°), since below this temperature iron is in a modification that is practically incapable of forming a solid solution with carbon. At temperatures lying between points Ac1 and Ac3, carburization of iron is possible, but the penetration of carbon is prevented by grains of ferrite, which persist in this temperature range (see Fig. 89, Fe-C phase diagram, section of the GOSP diagram).
When heated above the Ac3 point, carbon dissolution is much more intense.
In practice, the cementation temperature is in the range of 900-950°. Higher heating is harmful, as it leads to damage to the structure of the material, causes excess fuel consumption and faster wear of devices and devices used in cementation of products.
Carburizing agents (carburizing agents) can be solid, liquid or gaseous.
As a solid carburizer, they mainly use charcoal and animal charcoal with various additives: soda (Na2CO3), barium carbonate (BaCO3), etc. Impurities added to the main part of the carburizer affect the nature of carburization, enhancing it.
The enhancing effect of additives to coal is explained by the fact that solid carbon has a weak cementing ability, and its carburizing effect is stronger, the more carbon-containing gases it produces; additives to coal increase the gas content and thus enhance and accelerate the cementation process. For example, when heated, reactions occur
Carbon monoxide in the presence of iron decomposes into carbon and carbon dioxide according to the equation
the carbon released in this case is very active and penetrates the crystal lattice of y-Fe, forming a solid solution with iron.
Various cyanide compounds have greater carburizing ability; the effect of such additives to carburizers is also accompanied by the penetration of a certain amount of nitrogen into the iron, which increases the surface hardness of the product.
The carburizing mixture must be dry, thoroughly mixed and crushed.
Products to be cemented with solid substances are placed in boxes made of refractory material (cast iron, iron, fireclay, nichrome) and covered with carburizer so that the entire cemented surface is in contact with it. The grouting boxes, loaded in this way and carefully covered with clay along the cracks, are placed in the oven.
The result of carburization, other things being equal, will depend on the heating temperature of the carburized product and the time the product is kept at this temperature: the longer the carburization, the thicker the carburized layer. In fig. 121 shows a diagram of the dependence of the depth of carburization on the duration of the process for two types of solid carburizers at 900°. Approximately, we can assume that with an hour exposure, the depth of the cemented layer will be about 0.5 mm, with a three-hour exposure - about 1.5 mm, and with a ten-hour exposure - about 2.5 mm.
Liquid carburizers are used in cases where they want to quickly obtain a thin cemented layer with a high carbon content. Potassium cyanide mixed with borax, soda and other substances is often used as liquid carburizers. In this case, the product is either immediately immersed in a molten cement bath (t = 830°) or preheated to the cementation temperature and immersed in a crucible with salt heated to the same temperature. Sometimes a powder of a carburizing substance is poured onto a hot product, which melts and sticks to the surface of the product.
Using liquid carburizers for 15-20 minutes, a cemented layer with a thickness of 0.15-0.30 mm is obtained.
This method has a number of advantages, but requires careful handling of very poisonous cyanide compounds and their vapors.
Gas cementation is used mainly in the case of mass production of small products. Gas mixtures containing carbon monoxide (CO), ethylene (C2H4),
methane (CH4). The effects of these gases are different: hydrocarbons have
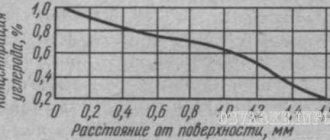
the effect is stronger. In fig. 122 shows curves characterizing the result of gas cementation; The depth of the cemented layer is plotted along the X axis, and the carbon concentration in it is plotted along the Y axis.
A good cementation result is obtained by using gas mixtures that include both hydrocarbons and carbon monoxide; in particular, cementation with illuminating gas gives good results.
Gas carburizing is carried out in rotating drums placed in special furnaces, where the drums with the products loaded into them are heated to the required temperature, a carburizing gas is passed through the drums, and the drums themselves are slowly rotated.
Since the process of carburization of the surface of products occurs at temperatures above the Ac3 point, and sometimes lasts up to 10 hours, the cemented product acquires a coarse-grained structure.
After carburization, the products are subjected to heat treatment, which consists of preliminary hardening or normalization from 900° and subsequent hardening from 750° C.
The first heating is carried out in order to impart fine grain to the core of the product. Since the carbon content in case-hardening steel does not exceed 0.2%, this heating should reach approximately 900° (see state diagram of Fe-C alloys, Fig. 89). Hardening (usually moderate in this case) imparts a fine grain to the core.
Due to the fact that the carbon content in the surface layer is much higher than in the core, and, therefore, its critical temperature is lower than for the core material, it will receive overheating during the first heating, and its structure after this operation will be coarse-grained.
To make the cemented layer fine-grained and hard, the product is subjected to a second heating, the height of which is determined by the carbon content in the cemented layer. With a carbon content close to 0.8%, the temperature of the second heating is close to 750-775° (see Fig. 89). This heating does not cause the formation of large grains in the core, since the position of the Ac3 point for the core is much higher.
After hardening, the cemented layer will have a martensitic structure; subsequent tempering (up to 200°) eliminates internal stresses in the product and regulates hardness.
In fig. 123 shows a micrograph of the structure of cemented steel before final heat treatment; The photograph clearly shows the change in carbon content from the periphery of the product to the core.
The result of cementation can be established with a sufficient degree of reliability by microscopic analysis, which makes it possible to trace the change in carbon content from the surface to the core. For this study, one of the products should be cut so that the cut plane is perpendicular to the surface of the product. The cut surface is ground and etched in a weak solution of nitric acid. The cemented layer under the influence of the etchant is painted dark gray, and the uncarburized part of the cut remains light.
The depth of carburization is sometimes controlled by a piece of steel that has the same chemical composition as the products being carburized. This piece, sometimes called a “witness,” is placed together with the products in a cementation box. After carburization, the control piece can be subjected to the same heat treatment as the products, cut and examined in relation to the results of carburization in any way. Obviously, the result of the analysis of the control piece will be the same as would be obtained during the analysis performed on the product itself.
Recently, the process of carburizing small parts has been successfully replaced by surface hardening with high-frequency currents, while using steel with a carbon content for the manufacture of parts at which hardening gives the necessary practical result (medium carbon steel).
Nitriding of steel products. Nitriding or nitriding of steel is the saturation of the steel surface with nitrogen.
The purpose of nitriding is the same as carburization—to obtain a hard surface in a product with a soft core. Nitriding was first used in Russia (N.P. Chizhevsky).
The nitriding process involves exposing steel to ammonia at a temperature close to 500-600°.
When heated, ammonia decomposes according to the equation
The resulting free nitrogen, which is in an atomic state, acts on the steel and forms, with the elements included in its composition (Fe, Cr, Al, etc.), various compounds - nitrides, which are distinguished by very high hardness.
Nitrides, as well as a eutectoid of nitrides and ferrite, are found in the nitrided layer. The structure of the eutectoid is similar to pearlite (Fig. 124); the nitrated layer obtained on alloy steels has high hardness, significantly exceeding the hardness of hardened steel.
Nitrided products do not require heat treatment, since the nitriding process occurs at temperatures not reaching the Ac1 point; the required hardness of the top layer of products is achieved without hardening due to the natural hardness of nitrides.
In fig. 125 shows a diagram of the nitriding installation; the ammonia cylinder 1 is connected through a reducer 2 and a gas flow meter 3 to an electric furnace 4. The nitrided products are placed in cage 5; the fan circulates gas in the furnace; pyrometer 6 is used to measure the oven temperature; The oil lock 7 in the furnace lid serves for sealing. Gas leaves the furnace through pipe 8. Equipment 9 is used to analyze the gas in the furnace.
The rate of penetration of nitrogen into steel is much lower than the rate of penetration of carbon; since nitriding is carried out at a lower temperature than carburization, and the mobility of the atoms of the dissolved element decreases sharply with decreasing temperature, the exposure time of the product in an ammonia atmosphere with appropriate heating ranges from 3 to 90 hours; it depends on the purpose of the nitrided products. The thickness of the nitrided layer is usually in the range of 0.25–0.75 mm.
Products subjected to nitriding undergo preliminary heat treatment - hardening and tempering up to 600° (to impart high mechanical qualities to the entire metal of the product), and then are saturated with nitrogen at 500-600°.
Nitriding of carbon steels does not give a sufficiently high hardness; Therefore, special steels containing 1.0–1.5% Al are usually subjected to nitriding. The approximate composition of one of the well-nitriding steels is: 0.3% C; 1.5% Cr; 1.0% A1; 0.2% Mo with normal Mn and Si content. Steels produced for nitriding are sometimes called “nitral”.
Nitrogen-saturated surfaces exhibit greater hardness compared to carburized ones; the increase in hardness during nitriding compared to the hardness acquired during carburization averages about 40%.
The abrasion resistance of nitrided surfaces is approximately 6 times greater than that of carburized surfaces.
Nitrided steels retain their hardness at temperatures reaching 500-550°, while carburized steels retain it only up to 200-250°.
High heating during carburization of steel causes significant deformations in it, leading to warping of products; during nitriding, due to the low heating temperature, the deformations are negligible.
Nitrided surfaces exhibit greater chemical resistance in air and in fresh and salt water.
These are the positive qualities of nitrided products; The negative aspects of nitriding are the need to use special steel and the duration of the process.
Types of chemical-thermal treatment of steel also include aluminizing, i.e. saturating the surface of steel products with aluminum, and diffusion chromium plating (saturating the surface of steel with chromium). The purpose of aluminizing is to increase the heat resistance and anti-corrosion resistance of steel and cast iron products; aluminizing processes can be carried out in various ways, in particular, by immersing the product in molten aluminum with some additives; Diffusion chromium plating is carried out by decomposition of chromium chloride vapor (CrCl2) due to the release of active metallic chromium on the surface of the workpiece at 950-1050° and its diffusion inside. Diffusion chromium plating is not yet widely used.
Download abstract: You do not have access to download files from our server. HOW TO DOWNLOAD HERE
Archive password: privetstudent.com