In the context of the topic of caring for the environment, the issue of keeping rivers and other bodies of water clean is often discussed. Now this is extremely difficult to do, because the wastewater that is discharged into water bodies is highly polluted.
After active participation in one or another industrial process, wastewater accumulates a huge amount of harmful elements, which, when released into an open body of water, lead to the death of aquatic inhabitants and plants, as well as other unpleasant consequences.
To measure the degree of pollution of wastewater, several indicators are taken as a basis, one of which is COD. what COD is and how to reduce this indicator in this material.
Why do we need indicators of the degree of wastewater pollution?
The amount of wastewater pollution can be identified by a number of indicators, the most common among them are:
- COD or chemical oxygen demand;
- BOD is its biochemical consumption.
Measuring an indicator such as COD is necessary to analyze the quality of wastewater or liquid in a reservoir or to study the state of waters in general. COD is a quantitative indicator ; it is one of the most informative and detailed.
The following substances act as wastewater pollutants:
- dissolved;
- weighted.
The method for studying the state of a liquid taking into account COD is to determine the amount of oxygen that was spent on the oxidation of organic matter and minerals containing carbon. COD is also called the unit of chemical oxidizability of water , since organic matter is oxidized by the action of oxygen. After all, it, in turn, is one of the most powerful oxidizing agents.
Oxidability, depending on the origin of the oxidizing agents, can be of the following types:
- iodate;
- bichromate;
- cerium;
- permanganate.
The most accurate indicators are determined by using the dichromate or iodate method . Oxidability is expressed as the ratio of the volume of oxygen that was spent on the oxidation of mineral and organic substances. It is expressed in milligrams per 1 square meter. dm. liquids.
It is necessary to purify wastewater in order to reduce the concentration of harmful substances to normal levels, which are approved in regulatory documents.
Cleaning is carried out at special treatment facilities or stations. Their layout depends on the quantity and quality of wastewater, as well as the level of its contamination. However, the wastewater treatment scheme will be the same and the main goal of the work is to reduce COD and BOD.
Impact of high COD and BOD values on humans and the environment
COD and BOD are the main indicators of the presence of organic matter in wastewater and when they are significantly exceeded, one can judge the high degree of pollution of the wastewater.
Firstly, insufficiently purified water containing difficult to oxidize substances and harmful toxic compounds causes the death of living organisms. The presence of excess nitrogen and phosphorus in water contributes to the onset of flowering of water bodies, the formation of neuro- and hepatoxins, which, when entering the human body, can cause diseases of the liver and central nervous system. The entry of poorly treated wastewater into the soil leads to the accumulation of various harmful chemical compounds in the soil layer, which negatively affect the fertile layer.
Secondly, a lack of oxygen leads to the death of fish and other living organisms, since the oxygen they need for breathing is consumed by bacteria to oxidize organic matter.
COD and BOD as criteria for water pollution
The COD value includes the total content of organic substances in the liquid in the volume of bound oxygen consumed for their oxidation. COD is a general indicator of industrial and natural water pollution.
But such an indicator as BOD determines the amount of dissolved oxygen that is spent on the oxidation of organic substances by bacteria in the required volume of liquid.
For identical samples, the COD value will be higher than the BOD value, since more substances are subject to chemical oxidation.
Difference between COD and BOD, permanganate oxidability. Ratio of COD and BOD. Calculation of oxidation
The content of organic substances in water can be assessed by various indicators:
- Permanganate oxidability
- COD
- BOD
To assess the content of organic substances in water, a strong oxidizing agent is added to it (this can be potassium permanganate, potassium dichromate, etc.). Organic substances enter into oxidation-reduction reactions (ORR) as reducing agents.
The oxidability of water is the amount of oxygen (mgO2/l) equivalent to the amount of a strong oxidizing agent spent on the oxidation of reducing agent impurities contained in a liter of water.
Example: It is necessary to determine the oxidability of acetic acid CH3COOH, i.e. the amount of oxygen required to decompose acetic acid into carbon dioxide and water.
CH3COOH + 2O2 → 2CO2 + 2H2O Molecular weight of acetic acid: 12+1+1+1+12+16+16+1 = 60 grams 60 grams of acetic acid require 2 (16+16) = 64 grams of oxygen
Fragment of the periodic table
In this case, the oxidability is 64/60 = 1.07 grams of oxygen per 1 gram of acetic acid.
1) Permanganate oxidability is an indicator characterizing the amount of oxygen (mgO2/l) equivalent to the amount of potassium permanganate KMnO4 (“potassium permanganate”) spent on the oxidation of reducing agent impurities contained in a liter of water.
In natural waters, the amount of organic substances significantly exceeds the amount of other reducing agents (nitrites, H2S, Fe2+), so the resulting oxidability value corresponds fairly closely to the actual content of organic substances.
When using permanganate oxidation, only easily oxidized organic substances are oxidized. Sometimes, when determining permanganate oxidability, only 50% of organic substances are oxidized; therefore it is also called partial oxidability.
Permanganate oxidability is used to assess the content of organic substances in natural waters in water treatment practice, because the content of organic substances in them is not high. For wastewater, the COD value is used.
2) Chemical oxygen demand (COD) is an indicator characterizing the amount of oxygen (mgO2/l) equivalent to the amount of potassium dichromate K2Cr2O7 consumed for the oxidation of reducing agent impurities contained in a liter of water. When determining COD, 95-98% of organic impurities are oxidized, which is why COD is called “total oxidability.”
COD is determined in wastewater treatment practice, because they are rich in organic substances.
The COD value is determined by heating organic compounds with chemically pure concentrated sulfuric acid, to which potassium iodate or chromic acid salts are added, which give up their oxygen for oxidation.
3) Biochemical oxygen demand (BOD) - the amount of oxygen (mgO2/l) consumed for aerobic oxidation to the stage of nitrification of organic impurities contained in a liter of water.
> in 5 days - BOD5 (within 5 days under normal conditions ~ 70% of organic substances are oxidized
> in 20 days - BOD20 or BODtotal (in 20 days almost complete oxidation occurs)
The process of oxidation of organic substances with the participation of aerobic microorganisms occurs in two stages:
1. ammonification stage
2. stage of nitrification
The BOD indicator does not take into account organic matter used for bacterial growth, as well as persistent organic substances that are not affected by the biochemical process. Those. Not all organic matter can be accounted for by the BOD indicator, and therefore both BOD and COD are determined for wastewater.
Relationship between COD and BOD
For domestic wastewater, BODtotal is 86% COD ; however, many process waters have a COD that exceeds the total BOD by 50% or more.
For approximate calculations, it can be assumed that the total BOD is 0.6–0.8 COD.
What factors influence COD
There are a lot of factors that can affect the composition of harmful substances and the acidity of a liquid. One of the key factors is the set of biochemical processes occurring in the reservoir itself . As a result of these processes, substances react with each other and form new ones, which may differ in structure from the previous ones and have a different chemical composition.
These substances can enter the reservoir as follows:
- along with precipitation;
- together with domestic or industrial wastewater;
- with underground and surface wastewater.
Their structure and composition can be very different, in particular which of them can be resistant to oxidizing agents . Depending on this factor, you need to choose the most effective oxidizing agent for certain substances.
In surface waters, organic matter can be suspended, dissolved, or colloidal. Oxidability differs for filtered and unfiltered samples . Natural waters are less susceptible to pollution by organic matter of natural origin.
Surface waters have a higher degree of oxidation compared to such types of water as:
- underground;
- ground and others.
For example, mountain rivers and lakes have oxidation in the region of 2–3 mg per cubic decimeter, rivers fed by swamps - 20 mg/cubic meter. dm and flat reservoirs - from 5 to 12, respectively.
A significant factor that affects oxidation is seasonal changes occurring in hydrobiological and hydrological regimes.
Also, the oxidation of a reservoir can change under the influence of human activity; depending on the sphere of human activity, pollution of one type or another enters the reservoir.
Methodology for determining the COD level
The amount of oxygen required for the oxidation of organic and mineral substances containing carbon is calculated.
OXYGEN IS ONE OF THE STRONGEST NATURAL OXIDIZERS!
Oxidability includes four types:
- Iodate
- Bichromate
- Cerium
- Permanganate
Preference is given to the dichromate or iodate methods, since these types of oxidation are the most accurate.
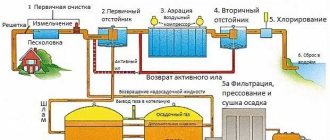
Stages of wastewater treatment and reduction of pollution levels
Wastewater treatment includes the following stages:
- Primary cleaning is the removal of oil films, large pieces of dirt and numerical contaminants that are easily removed. This stage involves cleaning using a physical-mechanical method;
- secondary cleaning . At this stage, suspended parts and pollutants, which are contained even in dissolved form, are separated. Some pollutants are organic in origin and must be removed through biological oxidation. This stage involves a biological method of wastewater treatment;
- tertiary treatment is the removal of all remaining small particles and contaminants, including metal salts. Purification is carried out by osmosis, electrodialysis, filtration through an adsorbent, etc.;
- fourth stage - at this stage the sludge is dewatered, which reduces its volume and weight to a minimum.
The level of COD and BOD is gradually reduced to certain values at each stage, the amount of their reduction depends on the characteristics of the wastewater.
Wastewater is not always treated in all four stages. Very often, treatment plants discharge wastewater into the collector after the first stage of treatment, and this brings the COD indicators back to normal . In some countries, purification is carried out in only two stages, the third stage being used only as a last resort.
What influences the level of COD indicators
The biochemical reactions that occur in water are the main factor. In the process, new substances appear that have a different chemical formula.
They can end up in liquid:
- With precipitation
- With domestic and industrial wastewater
- With surface and underground water flows
The structure of such substances may not be oxidizable. In such cases, the optimal type of oxidizer is selected. Natural reservoirs are highly oxidizable, unlike underground, groundwater, etc. water Mountain reservoirs have an oxidation rate of 2-3 mt per cubic decimeter. Rivers – 20 mg/cub.dm. Reservoirs located on the plain - 5-12 mg/cub.dm. Seasonal changes have an impact on oxidation. Human activities and wastewater disposal cause damage to oxidation.
The difference between domestic wastewater and industrial wastewater
Wastewater can be of industrial or domestic origin; the nature of the contaminants in them is also different. So, as a rule, household wastewater is contaminated with such things as:
- garbage;
- organic residues;
- detergents.
But industrial drains are filled with industrial waste; if it is the food industry, then there will be the most suspended solids and fats . COD and BOD values in industrial wastewater will be higher than in domestic wastewater.
Sometimes wastewater is combined, as a result of which organic matter from domestic wastewater becomes a breeding ground for activated sludge from bioremediation.
BOD to COD ratio
COD and BOD are measured simultaneously to provide information about the effluent. Data is compared and correlated. If the COD is higher than the BOD, this means that the water contains a lot of organic matter that does not oxidize.
The differing results are explained by the reactions that occur. Oxygen consumption varies depending on the type of process: chemical or biological. The ratio is influenced by the nature of the liquid and its contents. An increase in the difference occurs with insufficient biochemical oxidation. This means that liquid sewage is of little use for biological treatment.
Based on assessments of monitoring results, effective cleaning methods are selected.
COD standards
COD norm 15-30 mg/cubic dm:
- 2 mg/cubic dm – very clean water
- 3 mg/cubic dm – acceptable clean
- 4 mg/cubic dm – average water pollution
- 15 mg/cubic dm – we will pollute the reservoir
MPC (maximum permissible concentration) of harmful substances. MPC is the permissible content of a harmful substance that does not harm human health and his offspring and does not destroy the environment.
General water quality standards. Ion PKD g/m3:
- AI3+ 0.2
- Fe3+ 0.2
- Cu2+ 0.01
- Hg22+ 0.01
- Zn2+ 0.01
- Na+ 0.5
- SO42-20
- Cl-20
How COD levels are reduced and wastewater is treated.
Standards and methods for measuring BOD 5
If production or industrial wastewater (high content of difficult to decompose substances) is subject to research, then the conversion of BOD 5 to a full indicator is not applied. After taking the sample, it is incubated for 5 and 20 days (for industrial wastewater 120 days). Then it takes a measurement. Samples are taken daily for a specified time. If wastewater (usually household water) contains easily decomposed organic substances, then a conversion factor of BOD 5 to BOD is used, which is equal to 1.33, as indicated in the formula.
The results obtained are compared with the norm. For BOD 5, the standard is determined by GOST 2761-84. It stipulates that for sources of drinking (centralized) substances the indicator should be no more than 2 mgO2/l, for fisheries and cultural reservoirs the value should be no more than 3.5-4 mgO2/l. To maintain the BOD indicator within acceptable limits, maintain the ratio of COD to BOD 5 in household wastewater in the range of 0.4-0.75. The optimal value is 0.7. With this ratio between the indicators, the anaerobic purification process takes place optimally and in full.