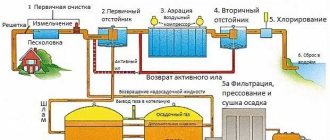
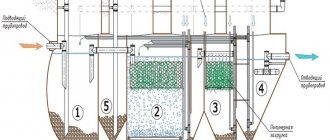
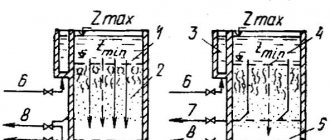
One of the regulatory documents that determines the quality of operation of sewerage systems is SanPiN sewerage. Along with the existing joint venture, it regulates the conditions and standards for the operation of networks, and sets standards for the content of harmful components in wastewater.
Without clearly defined rules and regulations, it is impossible to organize the effective operation of wastewater systems and obtain the desired results. Considering the direct influence of the quality of sewerage and treatment facilities on the composition of drinking water, one can understand the importance and severity of the requirements. Let's take a closer look at the regulatory documents and their requirements.
What is SanPiN
The abbreviation SanPiP stands for “Sanitary Rules and Norms”. Each industry or economy has its own requirements. For sewerage systems, there is a general SanPiN “water supply and sewerage”, a regulatory document that defines the rules for the operation of water supply and sewerage systems. This is a list of requirements for the composition of waste liquids, the method of their transportation, the type of treatment and other stages of the functioning of sewerage complexes. They are the main criteria that determine the quality of operation of treatment systems. Guided by the requirements of SanPiN, they determine the permissible quantities of harmful components in the wastewater composition, and carry out routine or repair work.
In addition to the general requirements, there are separate regulatory documents on sewerage. They regulate the operation of specific systems operating at industrial sites or disposing of wastewater. The operating conditions of the complexes depend on the composition and characteristics of the components present in the wastewater. For example, regulatory documents on household sewerage determine methods of transportation and purification of liquids. The standards for the content of harmful components are indicated, and the rules for maintaining networks are listed. One of the important sections is the methodology for monitoring the state of water in drinking water bodies. Stations are organized to take samples and determine the amount of harmful components.
SanPiN for industrial sewerage determines the standards for the use of networks and limits the volume of harmful components in the composition of discharged waste. If the amount of impurities is too high, the enterprise is obliged to put into operation its own wastewater pre-treatment systems. After processing, they are sent to the regular sewer network. Maximum permissible concentrations (MPC) of all harmful or toxic components are indicated, and lists of substances prohibited for discharge are defined.
Standards for domestic wastewater disposal (page 2)
To calculate domestic water drainage from individual buildings of the complex, the flow of wastewater is taken in accordance with SNiP 2.04.01-85 “Internal water supply and sewerage of buildings” (Table 2).
Water drainage standards for individual residential, communal and public buildings in the town
| Name of wastewater sources | Meter | per day max drainage, l | per hour max drainage, l |
| Residential buildings: | 1 resident | ||
| with running water, sewerage without baths | 120 | 6,5 | |
| with running water, sewerage and baths with gas water heaters | 225 | 10,5 | |
| with centralized hot water supply | 300 | 15,6 | |
| with centralized hot water supply for buildings over 12 floors high | 400 | 20 | |
| Dormitories: | 1 resident | ||
| no showers (barracks) | 60 | 7,8 | |
| with shared showers | 100 | 10,4 | |
| Dormitories: | 1 resident | ||
| no showers (barracks) | 60 | 7,8 | |
| with shared showers | 100 | 10,4 | |
| Hotels: | 1 resident | ||
| with shared baths and showers | 120 | 12,5 | |
| with showers in all separate rooms | 230 | 19 | |
| Hospitals (hospitals) | 1 bed | 115 | 8,4 |
| Clinics, outpatient clinics (health posts) | 1 patient | 15 | 2,6 |
| Mechanized laundries | 1 kg dry laundry | 75 | 75 |
| Baths (basins on benches with washing in the shower) | 1 person | 180 | 180 |
| Administrative (headquarters) buildings | 1 working | 16 | 4 |
| Educational institutions and secondary schools (educational buildings) | 1 student | 20 | 2,7 |
| Children's nurseries with day care for children | 1 child | 105 | 18 |
| Dining rooms | 1 dish | 16 | 16 |
| Food stores | 1 workplace | 250 | 37 |
| Hairdressers | Same | 60 | 9 |
| Cinemas | 1 place | 4 | 0,5 |
| Clubs (Officers' Houses) | 1 place | 10 | 0,9 |
| Gyms, stadiums | 1 athlete | 50 | 4,5 |
| 1 viewer | 3 | 0,3 |
Domestic wastewater can also come from industrial (special) facilities located on the territory of the town, such as technical areas, repair shops, motor vehicles, power plants, boiler houses, etc. For industries with significant heat emissions (more than 84 kJ, or 20 kcal/h -m cubic) the norm for domestic wastewater disposal per person per shift is 45 liters, and for the rest - 25 liters. In addition, in industries associated with body pollution or requiring a special sanitary regime, it is planned to discharge 40 liters of shower wastewater per worker, or 500 liters per hour from one shower net, into the domestic sewer system. In industries associated with the release of large amounts of polluting dust and moisture, as well as when processing contaminated objects, shower drainage of 60 liters per worker is provided.
As is known, water consumption and, accordingly, water disposal do not remain constant over time. Changes in daily costs depend on the season. During hot periods, more water is consumed per day than during cold periods. Fluctuations in wastewater consumption by hour of the day depend on the daily water consumption regime: less water is consumed at night than during the day. Wastewater discharge may be uneven over the course of an hour, but these fluctuations are usually not taken into account in the process of determining wastewater flow rates.
Features of sanitary requirements for drainage lines
Sanitary requirements for sewerage are divided into several sections:
- household (household, household and fecal systems);
- industrial (technically, systems are divided into subtypes based on the content or consistency of waste);
- stormwater (discharge of rain and melt water from urban areas and industrial sites).
Along with the general requirements, each of these systems has its own rules and regulations. The main task is to control the condition of wastewater that is discharged into water bodies. They are sources of drinking water for populated areas, so control of the quality of wastewater begins long before it enters treatment plants.
There are several types of sewer networks:
- alloy;
- separate (full or incomplete);
- semi-separated.
The difference between them lies in the method of transporting wastewater to treatment plants. All-alloy technology is designed for joint transport of domestic, storm or industrial wastewater to treatment facilities. This technique is only suitable for settlements that do not have hazardous industries or large volumes of waste liquids.
If a settlement contains hazardous industries, SanPiN “water and sewerage” requires the use of a separate drainage system. One collector is built for household waste, and its own pipelines for industrial or storm waste. Wastewater treatment is also carried out by different complexes. This method requires high construction costs. But the quality of cleaning increases significantly. In addition, it becomes possible to use special treatment complexes to treat wastewater from different systems. Typically, waste from industrial sites has a stable composition, so certain treatment technologies can be used. The same approach can be used for stormwater or sanitary systems.
Semi-separate networks are designed for transporting domestic and rainwater. If the volumes are relatively small and the level of impurities is high, the liquids are sent to treatment facilities. However, during heavy rainfall, when the collector is filled primarily with rainwater, emergency discharge into water bodies without treatment is allowed. SanPiN for sewerage allows this option for the operation of complexes if they do not contain harmful or toxic components. The technique allows you to unload equipment, avoid overloading treatment facilities, and increases their efficiency.
Also read: Repairing a sewer pipe without dismantling: ways to repair it
Indicators of the degree of purification
One of the most important indicators of the degree of water purification is the total biological demand for oxygen (or total BOD). According to sanitary standards, this indicator of biological treatment of wastewater, which is supposed to be discharged into water bodies, should not be higher than 3 mg/l at a temperature of +20 degrees.
In other words, one liter of treated wastewater must contain less than 3 mg of oxygen to oxidize the remaining contaminants.
In addition, the treatment of industrial wastewater and, in some cases, domestic wastewater is determined by such indicators as maximum permissible concentrations (or maximum permissible concentrations) of contaminants for oil products, suspended particles and other elements.
However, treatment plants, both industrial and small local, often treat wastewater only to the total BOD level, which is 15-25 mg/l.
Effluent purified to this state can be discharged into the ground, where final (natural) purification occurs. BOD has its own value for each area.
Purification of wastewater from petroleum products is typical only for large industrial enterprises.
Standards for maintenance and repair work
Sewage is a developed engineering system that periodically requires repairs. The loads on pipelines or equipment are quite large, and problems that arise become noticeable at a later stage. Prevention does not always eliminate the failure of individual elements. Therefore, regulatory documents have been created for the repair of sewerage networks, which clearly define the order and priority of all activities.
Problems in the operation of drainage lines negatively affect the quality of wastewater treatment. Therefore, control and timely restoration of pipelines are strictly regulated by both technical and sanitary requirements. Any detected malfunction must be corrected immediately in accordance with instructions and technical regulations. Particular attention is paid to wastewater networks of industrial enterprises. If the technology involves aggressive compounds, toxic or active substances, the condition of the pipes is constantly monitored by special monitoring devices.
The condition of pipelines requires special attention. the appearance or growth of deposits on the pipe walls poses a threat to the operation of the entire line. Bandwidth decreases, network operation mode changes. With increasing layers, there is a possibility of blockages and complete blockage of the pipe lumen. Regulatory documents for flushing external sewers determine the criteria and frequency of measures for cleaning the network cavity from deposits. A specific cleaning method and the number of activities per unit of time are indicated. Particular attention is paid to pressure pipelines, the condition of which requires constant monitoring and control. An accident on a line under pressure will stop the sewer system for a long time and will require lengthy, expensive repairs. Such incidents cannot be allowed to occur, therefore special standards for flushing pipelines have been developed.
Water quality standards for water bodies
There are several criteria for water quality standards.
Odor as a water quality standard
Smells are a special kind of irritant for the nasal mucous membranes. In order to understand how strong the smell of water is, points are used. Water acquires a particular odor due to the volatile odorous substances present in it. Where do they come from? It’s simple: various organisms live in water, which empty themselves and decompose there; in addition, various dissolved substances constantly interact in water, and, of course, a variety of sewage (industrial, household, domestic) is often poured into reservoirs.
The smell of water depends on many factors: substances (that are dissolved in it), temperature, pH, pollution, living flora and fauna, hydrological conditions, etc.
| Determining the intensity of the odor of water | ||
| Odor intensity rating, points | Odor intensity | The nature of the odor |
| 0 | No smell | No noticeable odor |
| I | Very weak | A smell that is not noticed by the consumer, but is detected by an expert |
| II | Weak | The smell that the consumer detects when brought to his attention |
| III | Tangible | A smell that is easily noticeable may be a reason not to drink the water. |
| IV | Distinct | A smell that attracts attention and forces you to stop drinking |
| V | Very strong | The smell is so strong that it makes the water undrinkable. |
Color as a water quality standard
Water quality standards also take into account the color of the liquid. The intensity of the color depends on the amount of colored substances dissolved in the liquid. This indicator is measured in degrees on the platinum-cobalt scale. To determine the color intensity, the color of the test water is compared with the accepted samples.
Articles recommended for reading:
- Types of water filters and their characteristics
- How to install a water filter - useful tips
- How to drink water correctly: practical recommendations
The liquid in reservoirs gets its color due to humus and iron (III) substances dissolved in it. The greater their number, the “brighter” the color of the reservoirs. How much of these substances will be in the water is influenced by many factors: geological conditions, aquifers, soil characteristics, as well as how far swamps and peat bogs are from them, etc. Water discharged by industrial enterprises into a river or lake, may change their color.
The water of reservoirs can have different levels of color: from one to several thousand units on the scale.
There are two types of water color: “true” and “apparent”. The first appears due to the dissolution of various substances in liquid. The reason for the second is the presence of colloidal and suspended particles in the liquid, which are related differently (this is affected by the pH value).
Water quality standards allow the use for drinking purposes only of liquids that are no brighter than 35 degrees on the platinum-cobalt scale. In addition, the requirements for water quality in recreation areas are as follows: in a test tube with the liquid being analyzed (height 10 cm), the color of the water should not be determined.
Brightly colored liquid is not allowed for drinking by water quality standards, since it contains less oxygen, which negatively affects the development of biological organisms. It is thanks to oxygen in water that oxidation of iron compounds and humic substances occurs.
Read material on the topic: How to check water quality: 9 interesting ways and more
Turbidity as a water quality standard
In reservoirs, this indicator is affected by finely dispersed impurities caused by insoluble or colloidal substances. The degree of turbidity is expressed descriptively: weak opalescence, opalescence, weak, noticeable and strong turbidity.
Water quality standards prohibit drinking water whose turbidity exceeds 1.5 mg/dm3 of kaolin.
This water quality standard is determined turbidimetrically (i.e., light is passed through the sample), then the sample is compared with standard suspensions. The unit of measurement for water turbidity is mg/dm3 (if the standard suspension is kaolin) or in MU/dm3 (turbidity units per dm3 if the main standard suspension is formazine). These units are correlated as follows: 1.5 mg/dm3 of kaolin is equivalent to 2.6 IU/dm3 of formazine.
This method determines the turbidity of water with an unstable composition and content of finely dispersed substances. Examination of unfiltered water using this scheme reveals both colloidal and coarser particles.
Hydrogen index (pH) as a water quality standard
The content of hydrogen ions (hydroxonium; H3O+) in water bodies is determined by the following ratio of the concentrations of carbonic acid and its ions:
CO2 + H20 <=> H+ + HCO3- <=> 2H+ + CO32-
To determine the number of hydronium ions, a value is used that is calculated as the logarithm of their concentration with the opposite sign:
pH = -log[H+]
If the water contains little CO2 (usually surface water), then it corresponds to an alkaline reaction. pH is directly affected by the process of photosynthesis occurring in green aquatic fauna (absorbs CO2, releases OH-). Also, the amount of hydronium ions in water is affected by humic acids contained in soils.
If there are quite a lot of sulfates of various metals in the water, then this indicator is influenced by the hydrolysis of heavy metal salts:
Fe2+ + 2H2O => Fe(OH)2 + 2H+
In rivers, pH is usually 6.5–8.5, in precipitation 4.6–6.1, in swamps 5.5–6.0, in seas 7.9–8.3. The pH value changes with the season. Thus, in winter, the pH of rivers usually ranges from 6.8 to 7.4, and in summer – from 7.4 to 8.2. The pH level of natural waters is also affected by the geology of the watershed.
Standards for the quality of water to be used for drinking purposes and for fishing establish a pH value of 6.5 to 8.5. The same pH value should be in recreation areas.
The pH level is a very important water quality standard. The concentration of hydronium ions in water affects all processes occurring in the water of natural reservoirs. This value determines the development of living organisms located in a particular reservoir; the stability of various forms of migration of elements, the aggressiveness of the effect of water on metals and concrete, and the processes of transformation of various forms of biogenic elements depend on it. pH can reduce or increase the toxicity of harmful substances.
Natural bodies of water do not become acidic overnight. The first stage of acidification does not make significant changes in water quality, since bicarbonate ions neutralize H+ ions. The situation remains at the same level until the alkalinity of the water is reduced by 10 times (i.e., the pH should become less than 0.1 mol/dm3). The next stage of acidification is a pH level of no more than 5.5 all year round, that is, moderate acidity. During this period, the types of living organisms in the reservoir begin to change noticeably. In the third stage, the pH value becomes even lower - usually 4.5. This value is not affected even by precipitation with higher pH, which is explained by the presence of humic substances and aluminum compounds in water and soil.
Based on pH level, natural water bodies are divided into 7 groups.
| Groups of natural waters depending on pH | ||
| Group | pH | Note |
| Strongly acidic waters | <3 | The result of hydrolysis of heavy metal salts (mine and mine waters) |
| Acidic waters | 3=5 | The entry of carbonic acid, fulvic acids and other organic acids into water as a result of the decomposition of organic substances |
| Slightly acidic waters | 5=6,5 | Presence of humic acids in soil and swamp waters (waters of the forest zone) |
| Neutral waters | 6,5=7,5 | Presence of Ca(HCO3)2, Mg(HCO3)2 in waters |
| Slightly alkaline waters | 7,5=8,5 | Presence of Ca(HCO3)2, Mg(HCO3)2 in waters |
| Alkaline waters | 8,5=9,5 | Presence of Na2CO3 or NaHCO3 |
| Highly alkaline waters | >9,5 | Presence of Na2CO3 or NaHCO3 |
Read the material on the topic: Is it possible to drink tap water?