Metals are materials that never lose their relevance. They are widely used in everyday life and in industry. Of course, today a lot of different alternative substances have been invented, on the basis of which materials are obtained that are not inferior in quality to metals. However, it is impossible to completely replace them. It is difficult to imagine fences and gates, gratings, manhole covers, tools and much more from something else.
Although plastic, glass, silicone, polyethylene and polypropylene have become firmly established in modern human life, it is difficult to replace fundamental parts of structures, numerous parts of cars and other vehicles with any alternative to metals. She simply doesn't exist.
Metals in the Periodic Table
In the Periodic Table of Chemical Elements, metals occupy a leading position. Of the 117 elements known today, more than 90 belong to metals. All these elements have a number of characteristic features that allow them to be classified as metals:
- Capable of conducting electric current.
- They have thermal conductivity.
- Malleable, ductile, amenable to rolling into sheets and wire (not all).
- They have a silvery sheen (except for copper and gold).
In addition to general properties, each such element also has a number of specific ones, which makes it so popular.
Ferrous metals - what are they?
This class includes:
- iron and all its alloys;
- manganese;
- chromium;
- vanadium;
- titanium;
- actinides and uranium (thorium, plutonium, neptunium and others);
- tungsten;
- alkali metals.
That is, of the entire variety of these substances, ferrous metals account for a minority. Moreover, generally not the most common ones (with the exception of iron) are found in the earth’s crust and subsoil.
But despite the fact that ferrous metals are represented by such a small number of elements, they are very common and voluminous in production and processing. A lot of products, parts, and accessories are made from iron and its alloys.
Ferrous metallurgy is quite extensive and in demand all over the world. Iron mining and processing is one of the most advanced technical and economic problems in many countries of the world, including Russia.
History [edit | edit code]
Iron mining began at least two millennia BC. The production of pure iron and its alloys became possible thanks to the experience accumulated by ancient metallurgists in smelting copper and its alloys with tin, silver, lead and other fusible metals.
In ancient times, iron smelting was carried out in furnaces coated with clay or lined with stone. Firewood and charcoal were loaded into the forge. Air was pumped through a hole in the lower part of the forge using leather bellows. Crushed iron ore was poured onto a mixture of charcoal and firewood. The combustion of wood and coal was intense. A relatively high temperature was reached inside the forge [2].
Due to the interaction of coal and carbon monoxide CO, formed during the combustion of coal, with iron oxides contained in the ore, iron was reduced and accumulated in the form of dough-like pieces at the bottom of the forge. The pieces were contaminated with ash, slag, smelted from the ore components. This type of iron was called raw iron. It was necessary to remove impurities from it before starting to manufacture the products. The heated metal was forged and the remaining slag, impurities, etc. were squeezed out on the anvil. Individual pieces of iron were welded into a single whole. This method existed until the 12th-13th centuries.
When they began to use the energy of falling water and set the bellows in motion mechanically, it was possible to increase the volume of air supplied to the forge. The forge was made larger, its walls grew out of the ground, it became the prototype of a blast furnace - a domnitsa. The domnitsa were several meters high and tapered at the top. At first they were square, then they became round. Air was supplied through several tuyeres. In the lower part of the house there was a hole, covered with clay, through which the finished iron was removed after the melting was completed. Improvements in smelting technology and lining the walls of the furnace with natural refractory stone made it possible to significantly increase the temperature in the furnace. A liquid alloy of iron and carbon—cast iron—was formed at the bottom of the furnace. At first, cast iron was considered a production waste, as it was brittle (hence the English name for cast iron - pig iron
, pork iron). Later it was noticed that cast iron has good casting properties, and guns, cannonballs, and architectural decorations began to be cast from it [3].
At the beginning of the 14th century. They learned to make malleable iron from cast iron, and a two-stage method of metal production appeared. Pieces of cast iron were melted in small crucibles - furnaces, in which it was possible to obtain high temperatures and create oxidizing conditions in the area of the tuyeres. Thanks to oxidation, most of the carbon, manganese, and silicon were burned out of cast iron. At the bottom of the crucible, a layer of iron mass—kritsa—accumulated. The mass was contaminated with slag residues. It was removed from the crucible with tongs or a crowbar and immediately, in a heated state, subjected to forging to squeeze out impurities and weld into one strong piece. Such horns were called shouting ones. They had greater productivity than cheese-blown ones and produced metal of higher quality. Therefore, over time, the production of raw iron was discontinued. It was more profitable to obtain iron from cast iron than directly from ore. As the quality of iron improved, the need for it in agriculture, military affairs, construction, and industry also increased. The production of cast iron increased, blast furnaces increased in size, gradually turning into blast furnaces. In the XIV century. The height of blast furnaces already reached 8 m.
Read also: Template for facing masonry
The accelerated development of metallurgy began after the replacement of charcoal with coke. Deforestation to produce charcoal led to the fact that already in the 15th century. In England it was prohibited to use charcoal in metallurgy. The use of coke not only successfully solved the fuel problem, but also contributed to an increase in the productivity of blast furnaces. Thanks to the increased strength and good calorific value of coke, it became possible to increase the diameter and height of the furnaces. Later, experiments were successfully carried out on the use of blast furnace top gas to heat the blast. Previously, all gases were released into the atmosphere, but now the fire pit was closed and the exhaust gases were captured.
At the same time, the method of producing steel was also improved. The critical method could no longer satisfy the need for iron. Carbon gave steel strength. The carburization of cast iron was carried out either in the solid state or by fusion with cast iron in small crucibles. But such methods could not produce much steel. At the end of the 18th century. A new process appeared at metallurgical plants - puddling. The essence of the puddling process was that the firebox was separated from the bath in which the cast iron was melted. As the impurities oxidized, crystals of solid iron fell out of the liquid cast iron and accumulated on the bottom of the bath. The bath was stirred with a crowbar, a doughy iron mass (up to 50 kg) was frozen onto it and pulled out of the oven. This mass, the kritsa, was pressed under a hammer and iron was obtained.
In 1856, Henry Bessemer in England developed the most productive method for producing steel from cast iron - by blowing air through liquid cast iron in a converter lined with siliceous bricks on the inside. Cast irons with a high silicon content were processed in Bessemer converters. The process went quickly: 15-18 tons of cast iron were converted into steel within 15-20 minutes. To process cast iron with a high phosphorus content, Thomas proposed a converter with a lining of calcium and magnesium oxides [4].
In 1864, the first open-hearth furnaces appeared in Europe, in which the melting of cast iron and the oxidation of its impurities were carried out in hearth (reflective) furnaces. The furnaces ran on liquid and gaseous fuel. The gas and air were heated by the heat of the exhaust gases. Thanks to this, such high temperatures developed in the furnace that it became possible to have not only liquid cast iron on the bath bottom, but also to maintain more refractory iron and its alloys in a liquid state. In open-hearth furnaces, they began to produce steel of any composition from cast iron and use steel and cast iron scrap for remelting. At the beginning of the 20th century, electric arc and induction furnaces appeared. Alloyed high-quality steels and ferroalloys were smelted in these furnaces. In the 50s of the XX century. began to use the process of converting cast iron into steel in an oxygen converter by blowing the cast iron with oxygen through a tuyere from above. Today this is the most productive method of producing steel. In recent years, processes for direct extraction of iron from ore have become significantly improved compared to the past.
The development of steelmaking production also entailed the development of new equipment for hot and cold processing of steel. At the end of the 18th century. rolling mills appeared for squeezing ingots and rolling finished products. In the first half of the 19th century. began to use large steam and air hammers for forging heavy ingots. Last quarter of the 19th century. was marked by the emergence of large rolling mills and continuous rolling mills with electric drives.
History of the development of ferrous metallurgy in Russia [edit | edit code]
In Russia until the 17th century. iron production was artisanal in nature. Iron smelting was carried out by individual peasant families or several peasant households together. Houses were built on the lands of the Novgorod region, Pskov region, and Karelia. At the beginning of the 17th century. Blast furnaces appeared at the Gorodishchensky factories near Tula, and the construction of factories began in the Urals. In 1699 the Nevyansk plant was built. Rapid production of cast iron began under Peter I. The Demidovs built a colossal furnace in the Urals at that time, 13 m high, which smelted 14 tons of cast iron per day. Large land estates lying next to the plant were assigned to the plant along with the peasants, who were obliged to work at it for a certain time. Serfdom provided factories with labor for a long time. Good natural conditions - ore, forests from which coal was burned, abundance of water, the energy of which was used to drive various mechanisms - contributed to the rapid development of Russian metallurgy. Cast iron began to be exported abroad [5].
But in the 19th century. Serfdom became a brake on the development of production. European countries and the United States have overtaken Russia in the production of iron and steel. If from 1800 to 1860 the production of cast iron in Russia increased only twofold, then in England it increased tenfold, in France - eightfold. The owners of Russian factories, who had cheap labor at their disposal, did not care about the development of production, the introduction of technical innovations, or easing the working conditions of workers. Gradually, the old Ural factories fell into disrepair and stopped.
The Ministry of Finance, which was in charge of the mining and metallurgical industry, sought to introduce advanced technical achievements in the country, primarily British ones. Reports on the achievements of European industry, compiled by foreign “agents” of the Corps of Mining Engineers, were regularly published in the pages of the Mining Journal. For example, Russian metallurgists and industrialists learned about Neilson’s invention of blast furnace heating and many others just a few months after their announcement. For example, back in the 1830s, shortly after J. Neilson introduced his invention, Christopher Ioakimovich Lazarev, a representative of the famous Armenian family of industrialists and philanthropists, conducted successful experiments in the use of heated blast at the Chermoz plant in the Perm region. But even ready-made technical solutions were practically not in demand, since external demand for Russian iron dried up at the beginning of the century, after Great Britain began to provide itself with metal, and domestic demand was extremely low. The number of proactive, enterprising people capable and willing to introduce innovations was small, since most of the country's population did not have any rights, not to mention capital. As a result, even those innovations that were introduced by the most technically competent and enterprising factory owners were more a tribute to technical fashion than a real tool for increasing economic efficiency [6] .
Read also: How to make grounding yourself in a private house
The situation changed at the end of the 19th century. — there has been an upsurge in the ferrous metallurgy of Russia, especially in the southern regions (Ukraine). In 1870, the Russian merchant Pastukhov built a plant in the city of Sulin for smelting cast iron using Donetsk anthracite. In the town of Yuzovka (now Donetsk), the Yuzovsky metallurgical plant, the largest at that time, was launched. The metallurgy of the South received rapid development with the discovery of iron ore deposits in Krivoy Rog. In combination with the reserves of Donetsk coal, this became the basis for the development of the mining industry in the South of Russia. Unlike the factories of the Urals, the southern factories were equipped with larger units. Blast furnaces were loaded with coke and produced approximately six to seven times more pig iron per day than in charcoal-fired furnaces.
In 1870, the first open-hearth furnaces began operating at the Sormovsky plant in Nizhny Novgorod, and converters also appeared in steel foundries in the Donbass. In 1910, the first arc steel-smelting furnace was installed, and at the end of 1917, an electrometallurgical plant with several electric furnaces began operating near Moscow [5].
During the years of the Civil War, the development of metallurgy was suspended, and only in 1926 was the level of 1913 reached - the maximum pre-revolutionary steel production of 4.3 million tons. Ferrous metallurgy in the USSR received intensive development during the years of the first five-year plans. The world's largest plants were built - Magnitogorsk and Kuznetsk; , Krivoy Rog. Old factories underwent radical reconstruction: Dnepropetrovsk, Makeevsky, Nnzhie-Dneprovskny, Taganrog. New high-quality steel plants were built: Elektrostal, Dneprospetsstal. In 1940, steel production reached 18.5 million tons and rolled steel 13.1 million tons.
The Great Patriotic War caused serious damage to the southern factories of the USSR. Most of the equipment of metallurgical plants was evacuated to the East. In the shortest possible time, the production of the metal necessary for victory was launched in the Urals and Siberia. New factories were built, such as Chelyabinsk, production was expanded at the Kuznetsk and Magnitogorsk metallurgical plants, the exported equipment was installed at factories in Zlatoust, Nizhny Tagil, and Serov. New grades of armor and gun steel were mastered, and the production of the necessary types of rolled products was established. The country's metallurgists quickly created a base for building up all types of weapons, and already in 1943 the Soviet Union significantly surpassed the enemy in the production of tanks, guns, aircraft and other equipment. In the post-war years, the ferrous metallurgy quickly recovered from its losses. By 1950, the level of ferrous metal smelting was one and a half times higher than the pre-war level. All subsequent five-year plans are characterized by a consistent increase in production volumes and the construction of new factories and workshops. The largest plants were: Magnitogorsk, Novolipetsk, West Siberian, Krivoy Rog, Cherepovets, Chelyabinsk and a number of others. Oxygen converters with a capacity of up to 350 tons, 900 tons open-hearth furnaces, two-bath steelmaking units, 200-ton electric arc furnaces, and blast furnaces with a useful volume of 5000 m 3 appeared. Continuous mills were built to produce sheets, long products, pipes, and an installation for continuous casting of steel (UNPC). Special metallurgy of high-quality steels and alloys has been developed: processes for producing steel in electroslag, vacuum induction, vacuum-arc, electron beam, and plasma-arc remelting plants.
Methods such as treating liquid steel in a ladle with synthetic slag and argon, and vacuuming the liquid metal are widely used. In 1974, the USSR took first place in the world in terms of production of ferrous metals. During the XI Five-Year Plan, about 6 billion rubles were spent on technical re-equipment of the industry. Converters with a capacity of 350 tons were built. Electric furnaces with a transformer power of 60-80 MVA were built, and the capacity of continuous steel casting plants reached 20 million tons per year. New coke batteries, sintering plants, ore mining and processing plants were built, including the Kostomuksha Mining and Processing Plant, the Oskol Electrometallurgical Plant for the production of steel from direct reduced iron was put into operation, two electrometallurgical plants began operating in Belarus and Moldova with a capacity of 600 thousand tons of finished rolled products per year. . The decommissioning of old units, the operation of which is not economically feasible, continues. Considerable attention is paid to improving the quality of metal at all stages of its production. Much work has been carried out to improve the quality of preparation of iron ore raw materials.
Contribution of Russian and Soviet scientists [ edit | edit code]
Outstanding scientists played a major role in the development of domestic metallurgy [7].
- P.P. Anosov developed the fundamentals of the theory of production of cast high-quality steel.
- D.K. Chernov is the founder of scientific metallurgy; his works on the crystallization of steel have not lost their significance to this day.
- Academicians A. A. Baikov, M. A. Pavlov, N. S. Kurnakov created deep theoretical developments in the field of metal recovery, blast furnace production, and physicochemical analysis.
- V. E. Grum-Grzhimailo, A. M. Samarin, M. M. Karnaukhov laid the foundations of modern steel and electric steel production.
- Academician I.P. Bardin is known throughout the world for his works in the field of blast furnace production and the organization of scientific metallurgical research.
Deposits of ferrous metals on the planet
In terms of production scale, iron ranks first among all metals. Its mass content in nature, including in the earth's crust, amounts to billions. At the same time, according to experts, man has explored only one hundred billion tons to date.
If we talk about the world's deposits of ferrous metals, primarily iron, it should be noted that they are found on all continents, in all parts of the world, except for the points of the Far North. The distribution by country is approximately as follows (in descending order):
- Russia (about forty percent of all world reserves);
- Brazil;
- Australia;
- Canada;
- USA;
- China;
- India;
- Sweden.
Geography of the world's ferrous metallurgy, its raw material base. Development problems.
Ferrous metallurgy includes the extraction and preparation (beneficiation, etc.) of raw materials, production of cast iron, steel, rolled products and ferroalloys. (Ferroalloys are alloys of iron with so-called alloying metals (manganese, chromium, etc.), used in steel smelting as special additives to improve the quality of the metal.)
Depending on the completeness of the main cycle (smelting cast iron, steel and rolled products), ferrous metallurgy is divided into metallurgical plants (including all three stages), conversion enterprises (without smelting cast iron) and small-scale (shop) metallurgy. Particularly distinguished are enterprises with electrothermal production of steel and ferroalloys.
The raw materials for ferrous metallurgy are iron, manganese and chrome ores.
In general, approximately 1 billion tons of iron ore are mined annually in the world, of which more than half of global production comes from three countries - China (23%), Brazil (17%) and Australia (13%). Iron ore production in these countries is growing rapidly. Iron ore is also mined in large quantities in Russia, Ukraine, the USA, India, Canada, Venezuela, France, Kazakhstan, etc. Its largest exporters are Brazil and Australia, providing about 60% of world exports. Many countries of the world, including those producing iron ore - the USA, Great Britain, Italy, China, etc., import it. The largest importers are Japan, Germany, and the Republic of Korea. Combines, despite certain structural changes that have occurred in the industry, remain the main type of iron and steel enterprises in most developed countries. Full cycle ferrous metallurgy is characterized by high material intensity of production, i.e. high consumption of materials used in relation to the weight of the finished product.
The consumption of iron ore is especially high, and coking coal is somewhat less. To smelt 1 ton of cast iron, at least 1.5-2 tons of iron ore are consumed (the richer the ore is in iron, the lower its consumption), 1-1.2 tons of coking coal, and a total of 4-5 tons of raw materials and fuel. In this regard, countries and regions rich in iron and manganese ores and fuel have always been considered ideal places for the development of ferrous metallurgy - geoglobus.ru. For example, India, China, Kazakhstan, Australia, and the Donetsk-Dnieper region of Ukraine are distinguished by a combination of resources of iron and manganese ores and coking coal. But such a favorable combination of natural resources for ferrous metallurgy is rare, so many metallurgical regions and centers arose either near the development of iron ore (for example, in Lorraine in France, in the deposits of the Great Lakes in the USA, in the Alps of Italy, in Sweden, Brazil), or in places of coal mining (for example, the Ruhr region in Germany, Pennsylvania in the USA, Donbass in Ukraine, Kuzbass in Russia, etc.).
An additional and very large raw material base for ferrous metallurgy consists of scrap metal resources (depreciation scrap, metallurgical waste, etc.). The processing of metal scrap is associated with an excess of steel smelting over cast iron; it is more profitable (cheaper) to immediately smelt steel from scrap, bypassing the blast furnace (iron foundry) production.
The leaders in global steel production (approximately 700-750 million tons) are China, Japan, the USA, Russia, Germany, the Republic of Korea, Brazil, England, France, and Italy. Steel production is a branch of specialization of the economy of a number of other countries - Australia, Canada, South Africa, Sweden, Austria, Spain, Ukraine, Poland, and the Benelux countries.
In global steel production, the share of developing countries is constantly increasing (about 40% of steel is produced), especially the newly industrialized countries (Republic of Korea, Brazil, India, Mexico, Argentina, etc.).
Steel production in the CIS member countries, in particular in Russia, has sharply declined in recent years. However, Russia is the largest supplier of ferrous metals to the world market (about 15% of world exports). The United States imports ferrous metals the most.
Geography of non-ferrous metallurgy of the world: raw material base, diversity of industry structure, development problems.
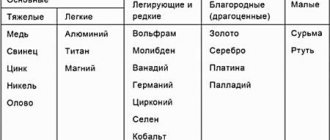
Non-ferrous metallurgy includes the production of non-ferrous, noble, rare metals and their alloys. In terms of production volume, the smelting of aluminum (more than 45% of the annual smelting of non-ferrous metals in the world), copper (25%), zinc (16%) and lead (11%) stands out. The production of nickel, tin, magnesium, cobalt, tungsten, and molybdenum is significant.
Non-ferrous metallurgy is distinguished by some features that affect its placement.
1. High material intensity of production, which makes it unprofitable to separate processing from the sites of extraction of raw materials. The percentage of most non-ferrous metals in ores is small (usually from a fraction of a percent to several percent), which predetermines the “linking” of ore processing enterprises to the places of extraction of raw materials.
2. High energy intensity of production, which makes the development of the industry effective based on sources of cheap fuel and electricity. Since the production (smelting - geoglobus.ru) of metals from enriched raw materials requires large amounts of energy, the stages of enrichment and metallurgical processing in non-ferrous metallurgy are often geographically separated.
3. The complex nature of the raw materials used. Many non-ferrous metal ores are polymetallic in nature, i.e. contain several metals. In order to completely extract (use) them in non-ferrous metallurgy, production combination is effective.
4. Widespread use of secondary raw materials in the production of resources (in developed countries, 25-30% of copper and aluminum, up to 40-50% of lead are smelted from scrap). For this reason, the location of non-ferrous metallurgy industries in many cases is focused on secondary raw materials (scrap metal).
Different branches of metallurgy have their own placement characteristics.
The leading branch of non-ferrous metallurgy (in terms of production volume and use of products) in the modern world economy is the aluminum industry. Among other branches of non-ferrous metallurgy, this industry is characterized by the greatest complexity of production. The first stage of aluminum production - the extraction of raw materials (bauxite, nepheline, alunite) - is focused on rich deposits. The second stage - the production of aluminum oxide (alumina), - being material- and heat-intensive, tends, as a rule, to sources of raw materials and fuel. And finally, the third stage - electrolysis of aluminum oxide - focuses on sources of cheap electricity (large hydroelectric and thermal power plants).
The main raw material for the production of alumina is bauxite, the world production of which is approximately 150 million tons per year. The vast majority of bauxite production and exports occur in Australia (almost a third of world production), Guinea, Jamaica, Brazil, China, India, Russia, Suriname, Greece, Venezuela, Kazakhstan. Most of the raw materials (approximately 2/3) are processed into alumina locally - in Australia, Brazil, Russia, Kazakhstan, etc. Part of the raw materials (approximately 1/3) is exported to countries where the main factor for the production of aluminum oxide is the availability of mineral fuel (local or supplied from outside) - USA, Canada, Ukraine , Ireland, Sardinia (Italy), etc.
The production of aluminum metal has been predominantly developed in countries with large sources of cheap energy - large hydro resources and powerful hydroelectric power plants (USA, Russia, Canada, Brazil, Norway, etc.), rich in natural gas (Iraq, Bahrain, UAE, the Netherlands, UK, etc. .) or coal (Australia, India, China, etc.). In some old, traditional centers of aluminum smelting (France, Austria, Hungary, etc.), where energy is expensive, its production has been greatly reduced and is gradually disappearing.
The largest aluminum producers in the world are Russia, USA, Japan, Germany, Italy.
The copper industry in its location is mainly focused on copper resources (natural and secondary raw materials). The low metal content in copper concentrates (from 8 to 35%) and the relatively low energy intensity of their processing (compared to aluminum smelting) make it profitable to locate copper production (smelting) in places where copper ores are mined and enriched. Therefore, copper mining and smelting sites are often geographically combined - geoglobus.ru. The main copper mining areas are in North and Latin America (Chile, USA, Canada, Peru, Mexico), Africa (Zambia, Zaire), CIS (Russia, Kazakhstan), Asia (Japan, Indonesia, Philippines), Australia and Oceania (Australia , Papua New Guinea).
The main copper-mining countries also stand out in terms of copper smelting; the leading place belongs to the USA, Chile, Japan, China, Canada, and Russia. Part of the mined ore in the form of concentrates and blister copper is exported to other countries (from Papua and the Philippines to Japan, from Latin American countries to the USA, from African countries to Europe, from Russia and Kazakhstan to Europe and China). Almost 1/5 of the world's copper smelting is based on scrap metal resources. The copper smelting industry of Great Britain, France, Germany, Belgium and other countries produces only secondary metal.
The zinc and lead industries usually have a common raw material base - polymetallic ores. Countries with the largest deposits of polymetals (USA, Canada, Mexico, Peru in North and Latin America, Ireland and Germany in Europe, Russia and Kazakhstan in the CIS, China, Japan, Australia) are also distinguished by their production. In terms of the volume of lead and zinc smelting, the leading positions in the world are occupied by economically developed countries of the world - the USA, Japan, Canada, Australia, Germany, France, Italy. China is a very large producer of lead and zinc. Russia is not among the top ten leading countries in global zinc and lead production.
The modern geography of the industry is characterized by territorial disunity of places of extraction and enrichment of lead and zinc ores and their metallurgical processing. For example, Ireland, which mines zinc and lead ores, does not have the capacity to smelt them, while in Japan, Germany, and France, the amount of metal smelting significantly exceeds the amount of zinc and lead production in these countries. Along with the influence of other factors, this is explained by the possibility of using long-distance raw materials, since the transportability of zinc and lead concentrates due to their high metal content (from 30 to 70%) is exceptionally high.
Location of the tin industry. The majority (about 2/3) of tin mining and smelting comes from the countries of Southeast Asia and, above all, Malaysia. Bolivia, Russia, and China also have large-scale tin mining and smelting.
In the world production of zinc, lead and tin, as well as in the copper industry, the share of secondary raw materials (scrap metal) is large. This is especially typical for the non-ferrous metallurgy of developed countries, where secondary raw materials provide 50% of the smelting of lead, 25% of zinc and tin.
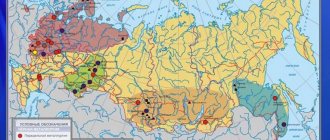
Deposits in Russia
In Russia, ferrous metals are found in almost all large federal districts.
- Central Federal District (Kursk Magnetic Anomaly) – over 59%.
- Ural Federal District – 14%.
- Siberian district - 13%.
- Far Eastern – 8%.
- Northwestern Federal District – 4%.
- Privolzhsky – 0.5%.
In each of the listed districts there is an enterprise that produces ferrous metals. Russia occupies a clear leading position in the world in this indicator, and, judging by its reserves, this will continue for a very long time.
Factors of location of ferrous and non-ferrous metallurgy
If we consider the general factors for the location of metallurgical industries, we can distinguish three types of metallurgical bases in Russia:
- a base that works with its own ore and coal;
- a base that uses either its own or imported ore, or works simultaneously with both types;
- a base that operates near coal deposits or close to potential and actual consumers
The main factors that influence the location of metallurgical centers are:
- consumer (proximity of large steel consumers - machine-building complexes);
- environmental (outdated enterprises that use a “dirty” method of manufacturing products - the blast furnace process);
- transport (due to the distance from fuel sources, enterprises use imported ore and coal)
- raw materials (enterprises that are located directly next to the location of the ore).
The basis for the development of mechanical engineering and metalworking is the ferrous metallurgy industry. Its products are used in almost all sectors of the economy. Russia is one of the top five global producers of ferrous metals along with the USA, Japan, China and Germany.
The production base of ferrous metallurgy consists of full-cycle enterprises: cast iron - steel - rolled products, as well as plants that produce cast iron - steel, steel - rolled products and separately cast iron, steel, rolled products.
Read also: The metalworking workbench is intended for
The factors for locating ferrous metallurgy enterprises are quite diverse. Special factors are highlighted in the production of electric steels and ferroalloys. Russia is sufficiently supplied with raw materials for ferrous metallurgy, but iron ores and fuel are unevenly distributed throughout the country.
Figure 1. Main factors for locating ferrous metallurgy enterprises. Author24 - online exchange of student work
Non-ferrous metallurgy in Russia is developing on the basis of the use of its own large deposits of various ores of non-ferrous, noble and rare metals, as well as diamond mining. The Russian Federation ranks second after the United States in the production of non-ferrous metallurgical industry products. The industrial structure includes 47 mining enterprises, of which 22 are in the aluminum industry. More than 70 different non-ferrous metals are produced in Russia.
In the territorial organization of non-ferrous metallurgy enterprises, the orientation towards raw materials is clearly expressed. This is due to the wide variety of raw materials and materials mined for the production of non-ferrous products.
Figure 2. Main factors for locating enterprises in the non-ferrous metallurgical industry. Author24 - online exchange of student work
Material extraction
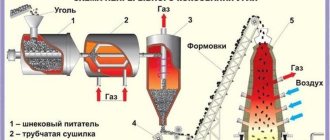
The production of ferrous metal involves several complex stage processes. Firstly, ferrous metals are not found in native form, but are part of the corresponding ores (manganese, iron, and so on). Therefore, before obtaining metal, it is necessary to extract rock - ore - from the earth.
This process is carried out by the mining industry. In this case, ores containing iron can be rich and saturated or poor in metal. Therefore, after extracting a layer of ore, a piece of it is taken for chemical analysis. If the quantitative metal content is over 57-60%, then work continues. If it is lower, then they stop or move to another territory to search for richer ore. Otherwise, this process is simply economically unprofitable.
The next stage, which includes the production of ferrous metal, is the processing of the extracted ore in a special plant. This process is called metallurgy. It can be of several types:
- Hydrometallurgy - the technology used to extract and process ore is based on the use of water. In this case, during the leaching process, metals from the ore go into solution, and from there they are extracted in pure form by electrolysis. This method is energetically and materially more expensive, therefore it is used only for special metals.
- Pyrometallurgy is the technique of using fire. Thermal treatment processes of ore in blast furnaces using coked coal. The most common method of processing ore and extracting metals. Used in ferrous metallurgy.
- Biometallurgy. It is based on the action of living organisms, it is just beginning to be introduced into practice, and is being developed by biotechnologists. The essence is the ability of some microorganisms to extract metals from ores during their life processes.
Peculiarities
Not only metals are the basis of ferrous metallurgy enterprises. Enterprises for the extraction and processing of related materials, coke, and refractories are also part of the ferrous metallurgy industry.
We can highlight the following features of ferrous metallurgy that are inherent specifically to it, in contrast to the production of non-ferrous metals:
- More than one third of manufactured products (steel and iron-based alloys, cast iron) are the basis of all mechanical engineering;
- More than a quarter of the products are used in construction to create elements of loaded and load-bearing structures.
The specificity of the enterprises of the metallurgical complex of ferrous metallurgy is that they, for the most part, form the basis of the state industry, being, at the same time, one of the highest capital and material intensive.
The organization of metal production at ferrous metallurgy enterprises is characterized by strong regional dependence. Processing ore and producing primary metal (pig iron) requires large amounts of coke, ore raw materials and electricity. It is estimated that raw materials and fuel account for more than 90% of the total ferrous metal production costs. The need to transport huge masses of ore and fuel raw materials dictates the need to solve problems of rational location of the enterprise. Most often, ferrous metallurgy enterprises are concentrated in this way:
- Near ore deposits. Fuel delivery required;
- Near fuel sources (coal mining enterprises). The question of supply of ore raw materials remains;
- At the optimal distance between sources of raw materials and fuel.
Most ferrous metal production plants are concentrated near iron ore deposits. This can be explained by the fact that initially, during the years of massive construction of metallurgical enterprises, the recovery of iron from enriched raw materials was carried out using charcoal mined directly near the deposits. When switching to the use of coke, it became more profitable to organize its delivery than to transfer metallurgical production.
It may be noted that the dependence of production on electricity is not indicated here, although the need for it is extremely high. This is explained by the fact that the transmission of electrical energy, even over long distances, is not comparable in complexity of organization and cost with the delivery of heavy and bulky production materials.
Enterprises for recycling ferrous scrap metal (conversion metallurgy) are concentrated near large mechanical engineering centers. Raw materials The raw material base is the basis of metallurgical production. Depending on the type of metallurgical enterprise, the sources of raw materials may be different. In particular, ferrous metallurgy can be divided into the following industries:
- Full cycle enterprises. Most stages of the production cycle, ore beneficiation, coke production, metal smelting and rolling are concentrated at one facility.
- Processing metallurgical enterprises. One of the stages, and this is mainly the production of steels and alloys, is separated into a separate industry.
- Small ferrous metallurgy. It is characterized by the fact that metal production shops are part of machine-building enterprises.
The raw material of ferrous metallurgy for processing and small enterprises is the semi-finished product for steel production - cast iron, scrap metal and other waste from the main metallurgical production. This group of industries includes the production of ferroalloys, which contain various alloying additives.
Ferrous metal ore mining
Ore mining, beneficiation, and smelting characterize full-cycle enterprises. Ferrous metallurgy is characterized by the use of raw materials with a high percentage of metal with large volumes of processing. Mining and beneficiation of ore requires significant expenditures of electrical energy and is demanding on the availability of available water resources.
Treatment
At the processing plant, the mined ores containing ferrous metals are carefully processed. All these processes are reflected in the table below.
The separation of a portion of the ore containing metal from the waste rock. Can happen in one of three ways:
- magnetic (based on the ferromagnetism of iron);
- gravitational (base – different densities of waste and rich rock);
- flotation (based on the use of water with a foaming agent).
Three types of processed ore are obtained:
- sinter ore (baked at high temperatures without air access);
- separated (purified by separation);
- pellet (mass containing iron fluxes).
| Technological process | The essence of the process | Result |
| 1. Ore beneficiation | A clean substrate rich in ferrous metal is obtained, which is sent for further processing. | |
| 2. Agglomeration | Ore sintering process. It is carried out with the aim of obtaining a pure substance, without impurities of gases and dust, and so on. | |
| 3. Domain process | Coking of ore in a blast furnace using coal as a fuel and reducing iron from its oxides. | Pure iron is obtained, if necessary already fused with carbon to form steel. |
This is how iron and its alloys are obtained. In this case, the maximum material costs are spent on the preparation and use of coke (coal). It is iron that is a reducing agent, a fuel, a source of heat, and a supplier of carbon. Therefore, in the described process, a fairly large amount of it is used, hence the high financial costs.
Heating, water supply, sewerage
Cast iron. Production. Pig iron is produced by smelting iron ore in blast furnaces. Modern blast furnaces have a volume of up to 5000 m3. The process of producing cast iron from ores is called blast furnace. To carry it out, ore, fuel and fluxes (fluxes) are loaded into the blast furnace shaft layer by layer. Coal coke is usually used as fuel, and limestone or dolomite as flux. As the coke burns, the ore and flux melt at a temperature of about 1500°. Molten pig iron accumulates on the blast furnace, and lighter blast furnace slag floats above the pig iron. As cast iron and slag are formed, they are released through special holes (tapholes) and sent for further processing.
Depending on the properties and name, cast iron is divided into foundry (gray), pigment (white), and special (ferroalloys).
The fracture of foundry cast irons is dark gray in color, which is why they are also called gray cast irons. Gray cast iron is produced in grades from SCh12-28 to SCh38-60, where the numbers indicate the tensile and bending strength, respectively, in kg/cm2. Compressive strength 0.5-0.75 kN/cm2. Cast iron is a brittle material.
The fracture of pig iron is white, coarse-crystalline. White cast irons are characterized by high hardness and good wear resistance. However, just like gray cast iron, they are brittle and cannot be processed with conventional cutting tools.
Special cast irons (ferroalloys) are an alloy of iron with silicon and manganese.
Gray cast iron is used mainly for the manufacture of structural elements operating in compression (columns, support pads, shoes, vaults, arches), as well as heating radiators, sewer pipes and fittings for them, slabs for floors of industrial buildings.
White cast iron is mainly used for steel production. In addition, it is used for the manufacture of parts that require high surface hardness and abrasion resistance (for example, steel rolls). White cast iron castings are also used to produce malleable cast iron, which has sufficient ductility, toughness and is easy to process. Special cast iron is used as additives in steel production to improve its quality.
Steel. Production. The starting materials for steel production are pig iron and steel scrap. Pig iron contains: carbon C up to 4%, silicon Si - 0.2-2%, manganese Mn - 0.6-3.5%, phosphorus P - 0.07-2%, sulfur S - 1 0, 06—0.08%. Due to the fact that steel must contain all these substances in much smaller quantities than cast iron, the main task when converting cast iron into steel is to remove most of these substances. Consequently, in order to obtain mild construction steel, it is necessary to increase the carbon content to 0.15-0.2%, reduce manganese by 2-4 times, and silicon by several times. It is especially important to reduce to possible limits the percentage of harmful impurities - phosphorus and sulfur.
Phosphorus causes cold brittleness in steels, that is, brittleness at low temperatures, which manifests itself more often at high carbon contents.
Sulfur causes red brittleness, i.e. brittleness at high temperatures, which causes cracks in steel during hot rolling, forging and welding.
The amount of carbon and impurities is reduced by oxidation and converting them into compounds that do not dissolve in the molten metal, but float and pass into the slag and are removed with it from the metallurgical furnace.
There are three ways to produce steel: converter, open-hearth and electric melting.
The converter method of steel production involves blowing air through molten cast iron. With this method, a special furnace is used - a pear-shaped converter with a capacity of up to 100 tons, rotating on a horizontal axis (Fig. 11). There are holes in the bottom of the converter for air injection. Molten cast iron is poured into an inclined converter, filling only part of its volume. Then the converter is placed vertically, and air is blown through the holes in the bottom through the molten cast iron under a pressure of 0.15-0.2 MPa. Air oxygen, interacting with cast iron impurities (C, Si, Mn), oxidizes them and turns them into slag. Cast iron, losing some of these impurities, turns into steel.
The converter method is highly productive. The steelmaking process lasts 15-30 minutes.
The open-hearth method makes it possible to obtain steel of different qualities by processing various cast irons with the addition of cast iron and steel scrap (the so-called scrap) and even iron ores. Steel is smelted on the hearth of a fiery reverberatory furnace (Fig. 12), i.e., a furnace whose working space is limited by a roof that reflects the heat flow. This furnace design significantly increases the temperature in the working space where heated fuel (usually gas) is burned. Before entering the furnace, combustible gas and air pass through the nozzles (lattice) of the regenerators. The stack of regenerators is alternately heated by the heat of gases escaping from the furnace, and then gives up its heat to the combustible mixture. Thanks to the heating of combustible gas and air, the temperature in the furnace reaches 1700°.
Steel scrap, fluxes and cast iron are sequentially loaded into an open-hearth furnace. After starting the furnace, the metal and fluxes gradually heat up and turn into a liquid state. Fluxes and its oxide formed during the oxidation of iron enter into a chemical interaction with harmful impurities and convert them into slag, which floats on the surface of the steel. As slag and steel are formed, they are released from the furnace through special openings.
The advantages of the open-hearth method lie in the possibility of using steel scrap and obtaining high-quality steels of the required chemical composition and properties. Open hearth steels are used for the manufacture of the most critical building structures - trusses, crane beams, crane rails. However, no new open hearth furnaces are currently being built.
The electric melting method of steel production is the most advanced, since in this case there is very little air access into the furnace, a very high temperature is achieved, the process can be precisely controlled and the production of high-quality steels can be ensured.
The most widespread in electric smelting are electric arc furnaces (Fig. 13), in which high temperatures are created as a result of the formation of electric arcs between carbon electrodes and molten metal. An alternating current with a voltage of 110-150 V is supplied to the electrodes at a current strength of about 103 A.
In the production of high-alloy and alloy steels, melting is also carried out in high-frequency induction furnaces, which are powered by current from a high-frequency generator. The process of producing steel in electric furnaces is similar to the open-hearth method and differs from it in that fuel and air are not supplied to the electric furnace for its combustion. This steel is of high quality. However, its cost is much higher than converter and open-hearth, which is explained by the high energy consumption.
Read also: Mini woodworking machines for home business
The process of obtaining finished steel (steel products) is carried out as follows. The steel, molten by one of the methods, is poured into special molds, where it hardens and forms ingots. To obtain the desired products, ingots are processed by rolling (in rolling mills), stamping, forging, drawing or casting. The most widely used method is the rolling method.
Carbon, alloy and other types of steel are used in construction.
Steels are divided into separate types mainly according to their chemical composition. Thus, carbon steels are steels that, in addition to iron, contain carbon, manganese, silicon, phosphorus and sulfur. Steels in which, in addition to the indicated elements, additional, so-called alloying components (nickel, chromium, aluminum) are introduced to increase the mechanical properties are called alloy steels. The properties of each type of steel depend on the percentage of elements included in the composition. With increasing carbon content in carbon steels, their properties change significantly, i.e., ductility decreases, hardness and brittleness increase. Therefore, carbon steels are divided into the following types: low-carbon (carbon content up to 0.25%), medium-carbon (carbon content 0.25-0.6%), high-carbon (carbon content 0.6-2%).
In addition, carbon steels are divided into ordinary structural and tool steels. Ordinary steel has grades from StO to St7 with a compressive strength of 32-75 kN/cm2 and a yield strength of 19-31 kN/cm2; Structural steel in construction is used in two grades: Styu and steel 20 (tensile strength 36-54 kN/cm2); Tool steel is characterized by increased strength.
The properties of alloy steels also depend on the type and amount of alloying additives used. Alloy steel, in which the total amount of alloying additives does not exceed 2.5%, is called low-alloyed 2.5-10% - medium-alloyed, more than 10% - high-alloyed.
Alloy steels are also divided into structural, tool and special. In construction, the most widely used low-alloy structural steel grades are 09G2, 14G2, 09G2T, 15GS, 10G2S, 15HSND, YuKHSND and 10G2SD with a tensile strength of 44-54 kN/cm2 and a yield strength of 30-40 kN/cm2. In brand designations, the first two digits indicate the carbon content in hundredths of a percent, the numbers inside indicate the content of the alloying element in whole percent, the letters indicate the name of the elements. For example, the symbol of the alloy steel grade 25ХГ2С shows that it contains 0.25% carbon, 1% chromium (X), 2% manganese (G) and 1% silicon (C).
Structural steel is used for the manufacture of critical machine parts and structures; instrumental - for the manufacture of cutting, measuring and impact-stamping tools; steel with special physical and chemical properties - for the manufacture of special-purpose parts.
Depending on the tensile mechanical properties, all steels used for steel structures are divided into conditional strength classes. In table 2 provides a classification of steel used for the manufacture of building structures.
Application. Carbon steel of ordinary quality is used for the manufacture of load-bearing and enclosing structures of buildings and structures, pipelines, reinforced concrete fittings, ropes, bolts, rivets, screws, nails, machine parts, railway rails; high-quality carbon structural steel - mainly for the manufacture of structural elements of communication structures (masts, towers), as well as in mechanical engineering; carbon tool - for the manufacture of various cutting tools.
Rolled steel. Rolled steel is most widely used in construction. Rolling involves pressing a heated ingot or billet between the rotating rolls of a rolling mill, resulting in a product of the desired shape. Carbon steel is mainly used to produce rolled construction products. Rolled steel is usually divided into groups: section steel, shaped steel, sheet metal, reinforcing steel for reinforced concrete.
Sectional steel is produced in round, square, strip, broadband, thin strip and angle profiles.
Shaped products include profiles of complex cross-section, which include: I-beams of various types, regular and lightweight channels, trough profiles for shaft fastenings, profiles for sheet piles, window sashes, and rails.
Rolled sheets contain thin-sheet, thick-sheet, corrugated and corrugated sheets, roofing, rolled steel of different thicknesses (for the construction of sheet structures - tanks, gas holders).
The range of basic rolled steel is shown in Fig. 14.
The main method of producing ferrous metals is the extraction of pig iron from ore and its subsequent processing into steel. Scrap metal is also used to produce steel. In recent years, direct production of steel from iron ores has begun to develop.
Iron production. Cast iron is produced in blast furnaces by high-temperature (up to 1900 °C) processing of a mixture of iron ore, solid fuel (coke) and flux. Flux (usually limestone CaCO3) is necessary to transform the waste rock (consisting mainly of SiO2 and Al2O3) contained in the ore and ash from fuel combustion into a molten state. These components, fused with each other, form blast furnace slag, which is mainly a mixture of calcium silicates and aluminates, similar in composition to Portland cement.
A blast furnace is a very large engineering structure. The useful volume of the furnace is 2000...3000 m3, and the daily productivity is 5000...7000 tons. The charge is loaded into the furnace (Fig. 7.1) from above through device 3, and air is supplied from below through tuyeres 7. As the charge moves downwards, its temperature rises. Coke, burning under conditions of limited access to oxygen, forms CO, which, interacting with iron oxides, reduces them to pure iron, oxidizing to CO2. Iron melts and at the same time dissolves carbon in itself (up to 5%), turning into cast iron. Molten cast iron 9 flows to the bottom of the furnace, and molten slag 2, being lighter, is located on top of the cast iron. Cast iron and slag are periodically released through tapholes 1 and 8 into the ladle. For every ton of cast iron, about 0.6 tons of fiery liquid slag is obtained.
Blast furnace slag is a valuable raw material for the production of building materials: Portland slag cement, porous aggregate for concrete - slag pumice, slag wool, etc.
Cast iron is mainly (about 80 %) used for steel production, the rest of the cast iron is used to produce cast iron products.
Depending on the composition, white and gray cast iron are distinguished. White cast iron is hard and durable, containing a large amount of cementite; in gray, due to the presence of silicon, cementite is not formed and carbon is released in the form of graphite.
Steel production. Steel is produced from cast iron and scrap iron and special additives, including alloying elements, by smelting in open-hearth furnaces, converters or electric furnaces.
Rice. 7.1. Blast furnace diagram: 1 - tap hole for producing liquid cast iron; 2 - molten slag; 3 - boot device; 4 - gas outlet pipe; 5 - drops of molten cast iron; 6 — drops of molten slag; 7 - lance for air supply; 8 - tap hole for releasing molten slag; 9 - liquid cast iron
Steelmaking is a complex process consisting of a number of chemical reactions between raw materials, additives and flue gases. The smelted steel is poured into ingots or processed into billets using the continuous casting method.
Read also: Development of a welding process
Manufacturing of steel products. Steel ingots are a semi-finished product from which the necessary products are obtained using various methods. Pressure treatment of steel is mainly used: the metal is deformed under the influence of applied force, maintaining its acquired shape. There is virtually no waste when processing metal by pressure. To facilitate processing, the steel is often preheated. There are the following types of metal forming: rolling, pressing, drawing, forging, stamping.
The most common processing method is rolling; It processes more than 70 steel products.
When rolling, a steel ingot is passed between the rotating rolls of a rolling mill, as a result of which the workpiece is compressed, stretched and, depending on the profile of the rolling rolls, acquires a given shape (profile). Steel is rolled mainly in a hot state. The range of hot-rolled steel includes round, square, strip, equal-sided and unequal-sided angle steel, channels, I-beams, sheet piles, pipes, smooth and periodic profile reinforcing steel, etc.
When drawing, the workpiece is successively pulled through holes (dies) of a size smaller than the cross-section of the workpiece, as a result of which the workpiece is crimped and stretched. When drawing, a so-called hardening appears in the steel, which increases its hardness. Steel drawing is usually done in a cold state, resulting in products with precise profiles and a clean, smooth surface. The drawing method produces wire, small-diameter pipes, as well as rods of round, square and hexagonal cross-section.
Forging is the processing of hot steel by repeated blows of a hammer to give the workpiece a given shape. Forging is used to produce a variety of steel parts (bolts, anchors, staples, etc.).
Stamping is a type of forging in which steel, stretched under the blows of a hammer, fills the shape of the die. Stamping can be hot or cold. This method can produce products of very precise dimensions.
Pressing is the process of squeezing steel contained in a container through the outlet hole (hole) of the matrix. ]J The starting material for pressing is casting or rolled billets. This method can produce profiles of various sections, including rods, small diameter pipes and various shaped profiles.
Cold profiling is the process of deforming sheet or round steel on rolling mills. Bent profiles with different configurations in diameter are produced from sheet steel, and hardened cold-flattened reinforcement is produced from round rods using cold forming machines by flattening.
Storage conditions
Ferrous metals primarily include iron and its alloys. It should be understood that this is a very corrosion-resistant material. Therefore, storing ferrous metal requires compliance with certain rules, especially if we are not talking about structures and products, but about the so-called scrap ferrous metals (waste, broken products, sheets, rods, fittings, and so on):
- The room in which the material is located must be completely closed from moisture (rain, snow). The less moisture, the longer the shelf life.
- The warehouse area must be large; sheet structures of ferrous metals cannot be stored close to each other, as this will provoke early corrosion.
- All available material should be sorted by brand and size.
If these simple rules are followed, it will be possible to restrain the processes of destruction of the structure of metals for as long as possible.
Ferrous alloys
These include iron alloys, which are divided into several types:
- Steel. Ferrous metal fused with carbon gives this result.
- Cast iron. Initial cast iron, which is obtained in blast furnaces during the processing of ore, is completely unsuitable as a material for the production of instruments and household items. He's too fragile. It needs to be further processed by being infused with iron and carbon to create an excellent durable material. Other elements are also added to increase corrosion resistance and improve technical characteristics.
- Ferroalloys (silicocalcium, ferrochrome, ferrosilicon, silicomanganese). The main purpose of these alloys is to improve the technical characteristics of the final material.
Steel
The main place among all alloys of ferrous metals is given to steel. Today we have learned to achieve very significant results in the production of this material with predetermined important properties. This kind of alloy is the most important thing for industry that ferrous metals have provided. What steels are selected?
- Low carbon - used for the production of various tools.
- Stainless steel (pipes, refractory parts, cutting tools, welding equipment, etc. are made from them).
- Ferrite-chrome.
- Martensitic-chromium.
- Alloyed.
- Nickel.
- Chrome.
- Chrome vanadium.
- Tungsten.
- Molybdenum.
- Manganese.
It is obvious from the names that these are the components that are added to the mixture of iron and carbon in a certain ratio. This affects a significant change in the properties of the resulting materials.
Secondary metals
Unfortunately, no matter how much we would like, things cannot last forever. Over time, everything becomes unusable - it breaks, breaks, ages and goes out of fashion. This happens with structures made of ferrous metals. Steel, cast iron and other products and spare parts simply cease to be needed.
Then they are handed over to special enterprises that process raw materials that have become unusable. Now these are secondary ferrous metals. This is what metal products made of ferrous metals that are out of order and unnecessary in everyday life are called.
Those enterprises that collect scrap must comply with certain rules for its storage, removal and sale. The legislation of our country establishes GOST on this issue. Ferrous metals, as well as non-ferrous ones, are strictly controlled by law.
Recycled metals can be recycled and put back into production. It is for sale for such purposes that intermediary entrepreneurs buy ferrous scrap metals.
Today, ferrous metals are treated with due respect; they occupy leading positions in the market for relevant products.
Use in mechanical engineering
Steel and cast iron objects, parts, and various devices are widely used in mechanical engineering. They are in demand not only in the automotive industry, but also in the chemical and aviation industries, as well as in shipbuilding. All this is due to the special strength of these materials, their heat resistance and corrosion resistance. Ferrous metals are becoming the base material for the production of many types of products. Among the most common are the following:
- side covers of gearboxes;
- bearings;
- valves;
- fitting;
- bushings;
- pipes;
- cylinders of cars and other vehicles;
- gears;
- chain links on tractors;
- brake drums;
- carriages;
- casings and so on.
This list can be continued endlessly, because there are really a lot of products made of ferrous metals and their alloys.
Neutral
Neutral are materials containing large amounts of Al2O3 and Cr2O3. For example, chromium-magnesite, fireclay bricks.
At high temperatures, the furnace lining interacts with fluxes and slags. If acidic fluxes are used in a furnace that has a lining lined with the main refractory material, then during the melting process acidic slags are formed, which, interacting with the main lining, will destroy it. The same thing will happen if basic fluxes are used in a furnace lined with refractory materials made from acidic oxides. Therefore, in furnaces with an acid lining, acidic slags are used, and in furnaces with a base, basic slags are used.
Carbon materials containing up to 92% carbon in the form of graphite have high fire resistance. The materials are used in the form of bricks, blocks for laying the bottom of blast furnaces, electrolysis baths for producing aluminum, crucibles for surfacing copper alloys.
Ferrous metallurgy
- a branch of heavy industry that unites technologically and organizationally enterprises for the extraction and beneficiation of ore and non-metallic raw materials, for the production of refractories, products of the coke industry, cast iron, steel, rolled products, ferroalloys, steel and cast iron pipes, as well as further processing products (rail fastenings, white tin, galvanized iron), metal powders of ferrous metals [1].
It serves as the basis for the development of mechanical engineering (one third of the cast metal from the blast furnace goes into mechanical engineering) and construction (1/4 of the metal goes into construction) [ source not specified 383 days
]. The main raw materials for the production of ferrous metals are iron ore, coking coals and ores of alloying metals.
Application in other industries
There are several main areas in which ferrous metals are used:
- Chemical industry.
- Mechanical engineering.
- Production of special purpose furniture.
- Release of dishes.
- Production of structural parts.
This is, of course, not a complete list, but only the most common areas, which account for the vast majority of iron and steel products.
The content of the article:
Iron ore is the main raw material for the global metallurgical industry. The economies of different countries largely depend on the market for this mineral, which is why the development of mines is receiving increased attention all over the world.
Ore properties
Iron is a common chemical element in nature. Its content in the earth's crust is about 4.2%. But in its pure form it is almost never found, most often in the form of compounds - in oxides, iron carbonates, salts, etc. Iron ore is a combination of minerals with a significant amount of iron. In the national economy, the use of ores containing more than 55% of this element is considered economically feasible.
What is made from ore
The iron ore industry is a metallurgical industry that specializes in the extraction and processing of iron ore. The main purpose of this material today is the production of cast iron and steel.
All products made from iron can be divided into groups:
- Pig iron with high carbon concentration (above 2%).
- Cast iron.
- Steel ingots for the production of rolled products, reinforced concrete and steel pipes.
- Ferroalloys for steel smelting.
What is ore needed for?
The material is used for smelting iron and steel. Today there is practically no industrial sector that can do without these materials.
Cast iron is an alloy of carbon and iron with manganese, sulfur, silicon and phosphorus. Pig iron is produced in blast furnaces, where the ore is separated from iron oxides at high temperatures. Almost 90% of the resulting cast iron is marginal and is used in steel smelting.
Various technologies are used:
- electron beam melting to obtain pure high-quality material;
- vacuum processing;
- electro-slag remelting;
- steel refining (removal of harmful impurities).
The difference between steel and cast iron is the minimum concentration of impurities. Oxidative smelting in open-hearth furnaces is used for purification.
The highest quality steel is smelted in electric induction furnaces at extremely high temperatures.
Technologies and production safety
The pace of technological re-equipment of the Russian ferrous metallurgy exceeds other industrial sectors.
The modernization of basic processing units carried out in recent years has made it possible to reduce production costs, which is the main competitive advantage.
Energy efficiency and the need for resources have also increased, which has led to a reduction in energy costs in environmentally harmful open-hearth steel smelting, which is now produced at converter and electric furnace steelmaking facilities.
One of the pressing problems at this stage of metallurgy development is the rational use of natural resources and ensuring environmental safety. When operating equipment used in the production of ferrous metals, harmful emissions are released into the atmosphere, which negatively affects both the environment and human health.
In terms of air emissions, this industry is in third place, ahead of it only in the energy sector and non-ferrous metallurgy.
Among the main sources of pollution with harmful substances are crushing and grinding equipment, sintering machines, and pellet roasting machines. Places where loading and unloading operations and transfer of materials occur are also dangerous.
In cities where large factories operate that process, smelt and produce goods from this industry, there is a level of pollution in the air with various impurities with a high hazard class.
A particularly high concentration of impurities is recorded in Magnitogorsk, where ethylbenzene and nitrogen dioxide have alarming indicators, as well as a similar situation in Novokuznetsk with nitrogen dioxide.
An increase in production provokes an increase in waste discharges, that is, water pollution occurs. According to research results, every ninth cubic meter of wastewater generated during the operation of Russian industrial enterprises is waste from ferrous metallurgy.
Although this problem is quite acute, in the current situation of ever-increasing competition with producers from the CIS, large-scale work that requires serious financial investments aimed at solving environmental problems is unlikely. The importance of iron and steel industry often exceeds the importance of ecology in the country. Enterprises specializing in steel production rarely think about the cleanliness of the environment. That is why a company arises that specializes in checking the work of black enterprises.
What types of ores are there?
Ore differs in the concentration of the element it contains. It can be enriched (with a concentration of 55%) and poor (from 26%). It is advisable to use low-grade ores in production only after enrichment.
Based on their origin, the following types of ores are distinguished:
- Magmatogenous (endogenous) - formed under the influence of high temperature;
- Surface - settled remains of the element on the bottom of sea basins;
- Metamorphogenic - obtained under the influence of extremely high pressure.
Main mineral compounds containing iron:
- Hematite (red iron ore). The most valuable source of iron with an element content of 70% and a minimum concentration of harmful impurities.
- Magnetite. A chemical element with a metal content of 72% is distinguished by high magnetic properties and is mined from magnetic iron ores.
- Siderite (iron carbonate). There is a high content of waste rock, the iron itself is about 45-48%.
- Brown iron ores. A group of aqueous oxides with a low percentage of iron, with admixtures of manganese and phosphorus. An element with such properties is characterized by good recoverability and porous structure.
What does ore look like?
The type of material depends on its composition and the content of additional impurities. The most common red iron ore with a high percentage of iron can be found in different states - from very dense to dusty.
Brown iron ores have a loose, slightly porous structure of brown or yellowish color. Such an element often requires enrichment, but is easily processed into ore (high-quality cast iron is obtained from it).
Magnetic iron ores are dense and granular in structure, looking like crystals embedded in the rock. The color of the ore is characteristic black-blue.
How ore is mined
Iron ore mining is a complex technical process that involves diving into the depths of the earth to search for minerals. Today, there are two methods of ore mining: open and closed.
Open pit ore mining
Open (quarry method) is a common and safest option compared to closed technology. The method is relevant for cases where there are no hard rocks in the working area, and there are no populated areas or utility systems nearby.
First, a quarry up to 350 meters deep is dug, after which iron is collected and removed from the bottom by large machines. After extraction, the material is sent on diesel locomotives to steel and iron factories.
Quarries are dug using excavators, but this process takes a lot of time. As soon as the machine reaches the first layer of the mine, the material is submitted for examination to determine the percentage of iron content and the feasibility of further work (if the percentage is above 55%, work in this area continues).
Closed mining method
Mine (closed) ore mining is used only if it is planned to maintain the integrity of the landscape in the area where ore deposits are being mined. This method is also relevant for work in mountainous areas. In this case, a network of tunnels is created underground, which leads to additional costs - the construction of the mine itself and the complex transportation of metal to the surface. The main drawback is the high risk to the lives of workers; the mine can collapse and block access to the surface.
Metallurgical complex
Ferrous and non-ferrous metallurgy are two main areas of the metallurgical complex. It includes the entire life cycle of metal production, from the extraction and enrichment of raw materials to the production of the finished product - various metals and their alloys. The entire metallurgical complex is a set of such technological processes as: - mining and preparation for processing of ore raw materials (the following types of work can be distinguished here: mining, enrichment, agglomeration, obtaining the necessary concentrates, etc.); — metallurgical processing – the main purpose of this technological process is the processing of cast iron, steel, rolling of ferrous and non-ferrous metals, pipes, etc.; — production of alloys; — recycling of industrial waste, the basic principle of which is based on waste-free production.
The main difference between metallurgical production and others is the scale of work that is incomparable with other industries and the complexity of the production cycle performed. Thus, for the production of certain types of products, from fifteen to eighteen processing steps are performed in order to obtain the final product. At the same time, the metallurgical complex has the most well-established logistics system not only with other enterprises in the Russian Federation, but also in the CIS countries. Another feature of the metallurgical complex is that, compared to other industries, more people are involved and work in it than in other industries. Today in the world there is no larger-scale production than metallurgical production.
Ferrous metallurgy
The foundation for the development of all mechanical engineering is ferrous metallurgy. It covers such processes as: extraction and preparation of fuel, raw materials, auxiliary materials for the production of rolled products with further processing products.
Main production processes in iron and steel industry:
— mining, enrichment and agglomeration of iron, chromium and manganese ores; — production of steel, cast iron, blast furnace ferroalloys and rolled products; — production of electroferroalloys; — secondary processing of alloys; — coal coking; — production of refractories; — extraction of auxiliary materials (magnesite, limestone, etc.); — production of products for industrial purposes.
The main type of work in ferrous metallurgy is metallurgical processing (cast iron, steel, rolled products), while the rest of the production is secondary, but plays an important role in the metallurgical industry. Today, according to world statistics, Russia occupies a leading place in terms of concentration of ferrous metal production, ahead of the United States. Over two-thirds of all steel, three-quarters of cast iron and three-fifths of rolled products in the world are produced by Russian enterprises with an annual productivity of more than three million tons of each. The leaders of production in our country are eight large enterprises: Magnitogorsk (MMK), Chelyabinsk (ChMK), Nizhny Tagil (N, West Siberian (ZSMK), Novokuznetsk (NKMK), Cherepovets (CherMK), Novolipetsk (NLMK) plants. On the territory of the listed industrial plants produces over four-fifths of all steel (including all converter steel and almost all steel cast on continuous casters), 9/10 of all cast iron in the country and over 4/5 of rolled products. From year to year, these enterprises process two-fifths of secondary raw materials and over nine tenths of iron ore.
It is worth noting that ferrous metallurgy enterprises are larger than non-ferrous metallurgy enterprises. However, if modernized scientific and technological developments are used in non-ferrous metallurgy, then in ferrous metallurgy the process of introducing modernization into production is much slower. Basically, the entire modernization process is based on waste-free production, which allows reducing costs.
An integral part of all metallurgical production is combination. The greatest benefits are obtained from combining metallurgical processing with coal coking. In our country, more than ninety-five percent of all coke is produced at metallurgical plants. Today, the largest ferrous metallurgy enterprises, by the nature of their internal technological relationships, are metallurgical and energy chemical plants.
Manufacturing plants are a type of enterprise with a full production cycle, currently characteristic of industrialized countries. In the Russian Federation, such enterprises produce about nine-tenths of all steel, cast iron and rolled products. In addition, there are also factories that produce cast iron and steel, or steel and rolled products, as well as each of the listed metals separately.
Pipe metallurgy includes all enterprises without iron smelting. Enterprises with electrometallurgical production of steel and ferroalloys occupy a special place in terms of technical and economic parameters. In ferrous metallurgy, “small” metallurgy is also distinguished as a separate production, producing steel and rolled products at machine-building plants.
Of all types of industrial production, ferrous metallurgy with a full technological cycle is the most important regional-forming factor. Ferrous metallurgy, in addition to numerous industries associated with the disposal of various types of waste, iron smelting and coal coking, includes related industries.
Directly related to ferrous metallurgy:
1. Thermal electric power industry - in the ferrous metallurgy, the use of secondary energy resources, such as coke, coke breeze, blast furnace gas, is widely used to reduce the consumption of materials and energy per unit of production; 2. Metal-intensive mechanical engineering - since this is a production based on the use of metal as the main raw material;
The Kursk Magnetic Anomaly (KMA) is the main concentration of iron ore reserves in Russia (21.6 billion tons). Its main iron ore deposits are: Lebedinskoye, Yakovlevskoye, Mikhailovskoye. In addition, there are reserves of iron ore in Russia in the Urals - about seven and a half billion tons, within which the Kachkanar group of deposits (three and a half billion tons) is especially prominent.
Eastern Siberia ranks third in terms of iron ore reserves in the Russian Federation (about five billion tons) with the Korshunovskoye and Rudnogorsk deposits in the Angara-Ilimsk basin and the Abakan group of deposits. In fourth place are the Far East (four and a half billion tons), the Northern region (almost three billion tons), where deposits such as Eno-Kovdorskoye, Kostamuksha and others are known, as well as Western Siberia (about two billion tons).
Most of the manganese ores are located in Western Siberia at the Usinsk deposit. The chromite ore basin is located at the Saransk deposit, in the Urals.
Ferrous metallurgy, using a full production cycle, is highly dependent on economic feasibility to sources of raw materials and fuel bases or, finally, to points located in between.
“Small metallurgy” is based on the separation of one of the stages into a separate production or is associated with the processing of secondary raw materials. Particle metallurgy is located mainly in the centers of large machine-building bases. It represents machine-building workshops that are part of large complexes.
Despite the fact that there has been a significant breakthrough in metallurgy in the development of new sources of raw materials and fuel throughout the country, the Urals still remains a major metallurgical center of Russia. The other leading giants of the metallurgical industry remain the Far East, the center and Siberia. The region of the North closes the list of leaders.
Half of the cast iron, rolled metal and steel in Russia is produced on the Ural soil. Thermal electricity is also widely used on the basis of such resources as Karaganda and Kuznetsk, and also in the production process, raw materials supplied from Kazakhstan are used - Sokolovsko-Sarbay ore, and also KMA resources. The use and consolidation of the raw material base as the basis for the life of the enterprise is associated with the development of titanomagnetites (Kachkanarskoye deposit) and siderites (Bakalskoye deposit), the extraction of which accounts for 3/4 of iron ore reserves. Titanium magnetites have long been used in mining (Kachkanarsky GOK).
Enterprises with a full production cycle play a dominant role in the development of pigment metallurgy, which explains the excess of steel production over pig iron production by 1.5 times. Pipe metallurgy enterprises are located along the western slopes of the Ural Mountains, in cities such as Yekaterinburg, Izhevsk, Serov, Zlatoust and Lysva, etc.
In the Urals, the concentration of metallurgical production has been at the highest level for decades. For the most part, ferrous metallurgy is a city-forming industry, and many cities in the Urals are single-industry. Large centers of full-cycle production are Chelyabinsk, Nizhny Tagil, Yekaterinburg, Magnitogorsk and Novotroitsk. The development of ferrous metallurgy in these industrial centers began during the period of industrialization.
However, despite the fact that the Urals is rich in large giant enterprises, today there are many small factories producing more than 1/10 of cast iron and steel and more than 1/5 of all rolled products.
The qualitative profile of the Ural metallurgy largely depends on the specification of the raw materials resources of the region. A special position is occupied by the production of ferroalloys by the following methods: blast furnace in Chusovaya and electrometallurgical in Serov and Chelyabinsk, and pipe rolling in Pervouralsk, Kamensk-Uralsk and Chelyabinsk. It should be noted that the Urals are the only region in the country where natural alloy metals are smelted in Novotroitsk.
The center of Russia, unlike other industrial regions of ferrous metallurgy, was formed not so long ago, without giant enterprises on its territory. Thus, the main non-interconnected industries in the center of the country are the smelting of foundry iron and blast furnace ferroalloys in Tula and the production of steel and rolled metal from scrap metal in Moscow, Nizhny Novgorod, Elektrostal and other industrial centers. Small metallurgy in the central part of Russia has always achieved major development.
Today the Center remains the main metallurgical base of our country. The center produces more than 2/5 of all iron ore in the country, and in the production of ferrous metals it is located somewhere on the same level as the Far East and Siberia. For a long time now, there has been no gap in the stages of pig iron production in the Center.
The construction, reconstruction and expansion of full-cycle enterprises - the Novolipetsk and Novotulsky plants, made it possible to reduce the role of separate processing stages for steel and cast iron, thereby creating a combined production. Of course, the levels of combination at the country's central factories do not at all reach the level of the Urals, producing only small parts of the total volume of finished product production in comparison with the Ural giants. The production of the central plants is entirely based on imported raw materials - Donetsk coal and coke. The main resources of the enterprise are the KMA deposits. Scrap metal is of particular importance.
Iron ore is mined by open-pit mining. Ferrous quartzites are developed in large quantities, along with rich ores (Lebedinsky, Stoilensky and Mikhailovsky GOKs). The Yakovlevskoye field is being developed. The Kursk magnetic anomaly is the main source of raw materials not only for the factories of the Center, but is also supplied to enterprises in the Urals, as well as the North.
In the Kursk magnetic anomaly zone, metallized pellets are being produced. Thanks to this, electrometallurgy is being mastered without blast furnace processing at the Oskol plant. The production of such a specific product as cold-rolled strip is located at the Oryol Steel Rolling Plant.
The metallurgical core in Siberia, as well as in the Far East, is still in the process of its formation. The metallurgical base of Siberia today is slightly inferior to the Center in the smelting of steel and cast iron, but is completely superior to it in the smelting of rolled products. There are two large enterprises with a full production cycle in Siberia - the West Siberian Plant in Novokuznetsk and the large Kuznetsk Metallurgical Plant. In addition, the Siberian land is rich in processing plants located in Novosibirsk, Krasnoyarsk, Guryevsk, Petrovsk-Zabaikalsk and Komsomolsk-on-Amur. Also in Siberia, there is a ferroalloy smelting plant in Novokuznetsk.
The raw material base of Siberia is the iron ore of Mountain Shoria of Khakassia and the Angaro-Ilim basin (Korshunovsky GOK), and the main supplier of fuel is Kuzbass. The formation of metallurgical production in the Northern region began in 1960 with the foundation of the Cherepovets Metallurgical Plant. The metallurgical plant, like other plants, prefers to work on fuel raw materials. The main raw materials for the plant come from the Kola Peninsula (iron ore), Karelia and coking coal from the Pechersk basin.
It is worth noting that, at a distance from the metallurgical bases, if you look at the map of our country, you can find another of the largest pigment metallurgical plants. Thus, in the Southern Federal District there are conversion plants in such cities as Krasny Sulin, Taganrog, Volgograd. Relatively recently, a new processing plant began operating in Komsomolsk-on-Amur, in the Far East.
Non-ferrous metallurgy.
Non-ferrous metallurgy includes the following work: mining, beneficiation, metallurgical processing of ores of non-ferrous, precious and rare metals, including the production of alloys, rolling of non-ferrous metals and processing of secondary raw materials, as well as diamond mining. It is worth noting that the activities of non-ferrous metallurgy are aimed at creating higher quality structural materials through the use of the latest technologies of scientific and technological progress in their core activities.
The complexity of the structure of non-ferrous metallurgy is primarily due to the wide variety of raw materials used and the widespread use of non-ferrous materials in their industry.
According to their physical characteristics and intended use, non-ferrous materials are divided into the following groups:
1.
Basic metals: heavy (lead, copper, zinc, nickel, tin) and light (potassium, sodium, aluminum, etc.) and small (cadmium, arsenic, antimony, etc.) metals;
2.
Alloying metals: niobium, vanadium, tungsten, molybdenum, tantalum;
3.
Noble metals: silver, gold, platinum with platinum group metals;
4.
Rare and scattered: zirconium, gallium, indium, thallium, germanium, selenium and others.
The composition of the Russian non-ferrous metallurgy is very diverse. Perhaps no other country in the world has so many industries. This is how the main industries are identified: copper, lead-zinc, nickel-cobalt, aluminum, titanium-magnesium, tungsten-molybdenum, hard alloys, rare metals and other industries, separated depending on the type of products, as well as gold processing.
Non-ferrous metallurgy is characterized by the stages of the technological process:
— extraction and enrichment of feedstock; — metallurgical processing; — processing of non-ferrous materials.
The organization of closed technological schemes with multiple processing of intermediate products and disposal of various wastes is typical for non-ferrous metallurgy. Over time, subject to the laws of scientific and technological progress, the trend towards recycling and disposal of waste will only grow in order to create waste-free production. Currently, a huge breakthrough is already taking place to expand the limits of technological combination. Thus, in the production of non-ferrous metals it became possible to obtain related products: sulfuric acid, mineral fertilizers, cement and others.
Due to the high material intensity of the production process, non-ferrous metallurgy is based on a raw material base, which is why production is “tied” to the places of extraction of industrial raw materials.
The main feature of the ore of non-ferrous materials is the low content of useful components; the composition of the ore is multicomponent. Thus, in non-ferrous metallurgy there is a principle of complexity in the use of the resulting raw materials.
Analyzing the technological process in non-ferrous metallurgy, one can notice that production combination has become not an opportunity, but a necessity of production in order to maximize the extraction of useful components.
The effectiveness of combination in non-ferrous metallurgy is based not only on the principle of waste-free production, as in ferrous metallurgy, but also on the fact that many elements obtained during the processing process do not have their own deposits, but can only be extracted through careful processing of the metal. In addition, it should be emphasized that the raw material base of non-ferrous metallurgy is located in poorly developed areas, which requires additional costs for the development of deposits.
Huge industrial complexes are being formed in certain regions of the country - in the North, the Urals, and Siberia due to the fact that non-ferrous metallurgy has related industries with a number of heavy industry branches. This is due to the integrated use of raw materials and recycling of production waste.
Thus, in the production process of zinc and copper, sulfur dioxide is used, the use of which is possible by combining non-ferrous metallurgy with basic chemistry. There is also a combination of production processes of non-ferrous metallurgy not only with the chemical industry, but also with the construction industry, for example, during the processing of nephelines, when in addition to the isolation of aluminum, soda, and potash as finished products, cement is also isolated.
An important role is played by the fuel and energy factor, which, depending on the requirements for fuel and energy, is divided into fuel-intensive and electricity-intensive production.
Unlike ferrous metallurgy, non-ferrous metallurgy is characterized by a predominantly large number of options for the location of production, depending on the stage of the technological process or the scheme for obtaining non-ferrous and rare materials. Thus, raw materials and fuel and energy factors do not affect the location of production complexes in the non-ferrous metallurgy industries.
Thus, the copper industry gravitates towards proximity to raw material resources due to the low content of concentrates in the resources. In order to eliminate production downtime and, consequently, an increase in the cost of production, the copper industry is located in the Urals. The main deposits of ore types used in copper production are Krasnouralskoye, Revdinskoye, Blavinskoye, Sibaiskoye, Gaiskoye and other deposits. The reserve is cuprous sandstones, the main reserve of which is concentrated in Eastern Siberia – the Udokan deposit.
Copper-molybdenum ores are also found. Thus, the largest deposit of copper-molybdenum ore in our country is the Taimyr Autonomous Okrug: Oktyabrskoye, Talakhninskoye and Norilsk. Together, these deposits provide 2/3 of all copper-molybdenum ore mined in Russia. The Oktyabrsky deposit alone accounts for 57% of the ore. The next largest ore mining region is the Ural copper ore region (Volga and Ural federal districts. The richest sandstone deposit is Udokan, located in the Chita region.
Copper-nickel and polymetallic ores are used as auxiliary raw materials in non-ferrous metallurgy. The main concentration of polymetallic ore in Russia is Western Siberia - the Salair group in the Altai Territory, Eastern Siberia - the Nerchinsk group in the Trans-Baikal region, the Gorevskoye deposit in the Krasnoyarsk Territory, in the Far East - the Tetyukhinsky group in the Primorsky Territory. Nicol ores are mined in the Murmansk, Orenburg and Chelyabinsk regions and the Krasnoyarsk Territory.
In the Urals, there are separate enterprises for the production of black copper and its processing. The production of black copper includes the Krasnouralsk, Kirovograd, Sredneuralsk, Karabash and Mednogorsk copper smelters, and its processing includes the Kyshtym and Verkhnepyshminsk copper electrolyte plants.
The lead-zinc industry has a more complex structural and territorial character in contrast to the copper industry. It gravitates towards the locations of polymetallic ores - Kuzbass, Far Eastern Primorye, North Caucasus and Transbaikalia.
Lead and zinc ores have a high content of useful components, which means that they are more transportable in contrast to copper, which leads to the fact that the process of enrichment and metallurgical processing can be separated from each other. For example, in the Urals, in the production of zinc, not only local ore concentrates are used, but also those imported from other territories of the country. Similar cases apply to lead smelting.
An individual feature of the lead-zinc industry is the territorial independence of beneficiation and metallurgical processing. Another feature of the complex is that zinc and lead cannot be obtained simultaneously in pure form everywhere.
The degree of completeness of the technological process distinguishes the following areas separately:
for the production of lead and zinc concentrates without metallurgical processing - Transbaikalia; for the production of metal lead and zinc concentrates - Far Eastern Primorye (Dalnegorsk); for the production of metallic zinc and lead concentrates - Kuzbass (Belovo); for the joint processing of lead and zinc - the North Caucasus (Vladikavkaz); for the production of metallic zinc from imported concentrates - Ural (Chelyabinsk).
The nickel-cobalt industry is most closely associated with sources of raw materials, which is due to the low content of intermediate products (matte and matte) obtained during the processing of the original ores.
In our country, two types of ores are exploited: sulfide and oxidized. The former are mined on the Kola Peninsula (Nickel) and in the lowlands of the Yenisei (Norilsk), and the lands of the Urals are rich in oxidized ores (Orsk, Rezh, Verkhniy Ufaley).
The tin mining industry is distinguished by the fact that the metallurgical processing does not depend on the location of the sources of raw materials. Thus, the redistribution is focused on areas of consumption of finished products, or production is distributed along the routes of concentrates (for example, Novosibirsk). All this is due to the fact that ore enrichment products are highly transportable and the extraction of raw materials is, as a rule, dispersed over small deposits. The basis of our country's tin resource base is located in Eastern Siberia and the Far East. There are such large production facilities as Sherlovogorsky, Khrustalnensky, Solnechny, Esse-Khaisky and other mining and processing plants. Of particular interest is the geography of light metals.
Aluminum industry.
The aluminum industry itself is unique in that, unlike all other non-ferrous metallurgy, it uses materials of higher quality. The raw materials are bauxite and nepheline. The main places of bauxite mining are Boksitogorsk and Severouralsk. Please note that single-industry cities specializing in bauxite mining. Quite recently, a new bauxite deposit was developed - Severo-Onezhskoye, located in the Northern region.
Nephelines are mined on the Kola Peninsula in Kirovsk, as well as in Eastern Siberia in Goryachegorsk. In their composition, they differ from each other in that bauxite is a simple raw material, and nephelines are complex.
Let us highlight the main stages in the aluminum industry:
— alumina production; — production of aluminum metal.
Geographically, the stages can be located within the same area, but there are different location factors, according to which the processes exist separately. Alumina production gravitates towards the source of raw materials, being material-intensive, and the production of aluminum metal is energy-intensive, which in turn requires sources of mass and cheap electricity.
Regions where limestone and cheap fuel are found along with aluminum raw materials should be considered optimal for alumina production. These include, in particular, Achinsk-Krasnoyarsk in Eastern Siberia and North Ural-Krasnoturinsky in the Urals.
All aluminum production centers (with the exception of the Urals) are mostly remote from raw materials and located near hydroelectric power stations. This is due to the fact that aluminum production requires cheap fuel.
The final stage of the technological process in non-ferrous metallurgy - processing of metals and their alloys - is close to areas of consumption and is usually located in large industrial centers. Consumption areas also attract the processing of secondary raw materials - an important additional resource in increasing the production of non-ferrous metals, which makes it possible to obtain finished products at much lower costs.
The gold mining industry is one of the oldest industries in Russia. According to world statistics for 2009, the share of gold production in Russia is 8.9% of world gold production. The leading country in gold production is China - 12.8%, followed by Australia - 9.4%, and South Africa closes the top three - 8.9%. We are in 4th place in gold production in the world. The leader in gold mining in Russia is the Khabarovsk Territory. The lands of the Krasnoyarsk region, Irkutsk, Amur, Sverdlovsk and Magadan regions, the Republic of Sakha and Buryatia, and the Chukotka Autonomous Republic are also rich in gold.
Where is ore mined?
Iron ore mining is one of the leading areas of the economic complex of the Russian Federation. But despite this, Russia's share in world ore production is only 5.6%. World reserves amount to about 160 billion tons. The volume of pure iron reaches 80 billion tons.
Countries rich in ores
The distribution of minerals by country is as follows:
- Russia - 18%;
- Brazil - 18%;
- Australia - 13%;
- Ukraine - 11%;
- China - 9%;
- Canada - 8%;
- USA - 7%;
- other countries - 15%.
Significant deposits of iron ore have been noted in Sweden (the cities of Falun and Gellivar). In America, a large amount of ore was discovered in the state of Pennsylvania. In Norway, the metal is mined in Persberg and Arendali.
Ores of Russia
The Kursk magnetic anomaly is a large deposit of iron ore in the Russian Federation and in the world, in which the volume of unrefined metal reaches 30,000 million tons.
Kursk magnetic anomaly - photo from the mining site
The area of the Kola Peninsula mines is 115,000 sq. km. Iron, nickel, copper ores, cobalt and apatites are mined here.
The Ural Mountains are also among the largest ore deposits in the Russian Federation. The main development area is Kachkanar. The volume of ore minerals is 7000 million tons.
The metal is mined in smaller quantities in the West Siberian basin, Khakassia, the Kerch basin, Zabaikalsk and the Irkutsk region.
Metals are divided into non-ferrous and ferrous. Ferrous metals , in essence, are iron that contains varying amounts of carbon, as well as different crystal lattice. Ferrous metals include steel and cast iron, which in turn have a fairly large number of main classes. In the production of cast irons and steels of various types, ferrous metals extracted from metal ores are used. In the metal economy, ferrous metals account for more than 90%, indicating their wide distribution. The percentage of carbon determines what properties the material will acquire—cast iron or steel. To improve the quality of ferrous metal, alloying additives (other metals and alloys, as well as chemical elements) are used, which improve the properties of the alloys and give them the desired shade of characteristics depending on its application. Common alloying additives are:
Concept and branches of the metallurgical industry
The basic industry of the Russian Federation, which determines the viability of the economy, is the metallurgical industry. In addition, this is one of the key areas for the development of the country’s economy, since its share in GDP is 5%.
The metallurgical industry is a branch of heavy industry that involves the processes of making metals from ores or other materials, as well as metal alloys.
The structure of the metallurgical industry includes the following processes: direct production of metals; hot and cold processing of metal products; welding; application of metal coatings.
The procedure for manufacturing metal products itself consists of three stages: mining and preparation of ore; remelting; use and disposal.
Various raw materials are used in the metal production process. Depending on what kind of raw materials are used, ferrous and non-ferrous metallurgical industries are distinguished. The first category includes metals that include iron, manganese and chromium. The other group includes all other metals.
Try asking your teachers for help
The ferrous metallurgical industry refers to the extraction from the depths of the earth and subsequent processing of ferrous metal ores, as well as steel and iron foundries, rolling of billets and the production of iron alloys.
The products that are produced at metallurgical plants are: the main (or final product); by-product (or that which appears during the manufacture of main products); by-products (or those products that remain after the production of main and by-products and can be used as secondary raw materials).
The main products of the ferrous metallurgical industry are rolled metal, cast iron, hardware, etc.
If we compare ferrous with non-ferrous metallurgy, a lot of energy is spent in the production of non-ferrous metal products. This is explained by the low content of useful substances in non-ferrous metals and large volumes of waste, which require certain methods of disposal.
Ask a question to the experts and get an answer within 15 minutes!
The main types of non-ferrous products are long products and sheet metal.
Classification of ferrous metals
In most cases, the classification of ferrous metals is based on the division of elements according to their chemical composition and properties . The content of alloying elements determines the iron and its alloy. In turn, a certain percentage of carbon in the alloy indicates whether it is cast iron or steel. Thus, cast iron contains more than 1.7% carbon, and steel contains from 0.2 to 1.7% carbon. Classification of ferrous metals involves division into the following classes:
- iron metals;
- refractory;
- uranium;
- alkaline earth;
- rare earths.
Also, the classification of ferrous metals implies the separation of alloyed and unalloyed steels, which are also called carbon steels. Carbon steels include steels in which carbon is the main component, and impurities do not have much effect on the properties of the metal. Alloyed steels have one or more alloyed elements, which have a huge impact on the properties of steel. /Alloy steels are very widely used for the manufacture of critical parts that bear heavy loads, experience different temperatures, and strong frictional effects. The use of such steel is widespread in mechanical engineering, tractor manufacturing, heavy industry and other areas.
Types of ferrous metals
Types of ferrous metals made from steel have many uses. However, all types are different and have their own purpose and area of application. Also, various types of ferrous metals , in particular steel, after undergoing heat treatment, acquire distinctive properties. Many alloys lend themselves well to rolling, pressing, and casting successfully. Others are soft enough to be processed by hand. Such types of ferrous metals as stainless steel, having the necessary alloying elements, have very high resistance to corrosion, great hardness and strength. This type of steel is successfully used in the food industry, medicine, for the manufacture of household items, for the production of turbines, etc. Another type of ferrous metal is cast iron. Cast iron is an alloy of iron and carbon and its content is higher than in steel. Since cast iron has good casting properties, it is mainly used for cast parts. Cast iron is divided into types:
- Foundry cast iron;
- Pig iron;
- Anti-friction cast iron;
- Ductile iron;
- Low alloy cast iron;
- High alloy cast iron;
- Nodular cast iron;
- Cast iron with vermicular graphite for castings.
Foundry cast iron is used for casting; flake graphite contributes well to this. Malleable - has remarkable ductility, can be forged well, which is where the name comes from. Certain types of ferrous metal , for example, nodular graphite cast iron, due to its spherical structure, are used in the manufacture of parts of very high quality.
Ferrous and alloy metal ores
Ferrous metal ores are part of all igneous and sedimentary rocks, but the name ferrous ores refers to such accumulations of ferrous compounds from which metallic iron can be obtained in large quantities and with economic benefits. Iron ores are found only in limited areas and only in known areas. In terms of their chemical composition, they are oxides, hydrates of oxides and carbon dioxide salts of ferric oxide, found in nature in the form of various ore minerals, the most important of which are: magnetic iron ore or magnetite, iron luster and its dense variety red iron ore, brown iron ore, which includes swamp and lake ores, and finally, iron ore in its variety spherosiderite. Typically, each accumulation of the named ore minerals is a mixture of them, sometimes very close, with other minerals that do not contain iron, such as clay, limestone, or even with components of crystalline igneous rocks. Sometimes some of these minerals occur together in the same deposit, although in most cases one is predominant, and the others are genetically related to it.
The beginning of the use of iron dates back to the 3rd millennium BC, when people made labor and hunting tools and jewelry from meteorites. In the 1st millennium BC. people began to smelt iron from ores, the Bronze Age was replaced by the Iron Age. With the development of metallurgy, brown iron ores began to be smelted in blast furnaces, first with charcoal, and from the 19th century. on coal and coke. They learned to smelt steel from cast iron. And in the twentieth century. and high-quality alloy steels by adding manganese, chromium, titanium, nickel, cobalt, vanadium, tungsten, molybdenum, niobium, tantalum.
Alloying metals include: manganese, chromium, titanium, vanadium, nickel, cobalt, molybdenum, tungsten are mainly used as alloying additives for the manufacture of alloy steels.
Manganese
Manganese ores have been used since the end of the 18th century. for the production of paints and medicines. In connection with the development of ferrous metallurgy, manganese ores began to be widely used in the second half of the 19th century.
Currently, metallurgy is the main consumer of manganese. The addition of manganese increases the viscosity of steel, its hardness and malleability, and promotes the transfer of many harmful impurities into slag. Manganese is used in small quantities in the electrical, chemical and ceramic industries.
Chromium
Chromium-containing ores were first discovered in the Urals in 1799. At the beginning of the 19th century. they were used as a refractory material for lining metallurgical furnaces, producing paints and leather tanning agents. At the end of the 19th century. chromium began to be widely used as an alloying metal. Currently, the main consumer of chromium-containing ores is the metallurgical industry (65%), the rest are used in the refractory and chemical industries. Chromium is used for the production of stainless, heat-resistant, acid-resistant, tool and other steels.
Titanium
Titanium was discovered in 1791, but began to be used only in the middle of the 20th century. The properties of titanium are unique: melting point 17250.
Titanium is characterized by high strength and corrosion resistance. Titanium alloys, characterized by high strength, malleability and weldability, are used in space technology, aviation, automotive, shipbuilding, food and medical industries. Titanium carbide is used for the production of super-hard alloys, titanium dioxide for the production of durable titanium white, plastics and in the pulp and paper industry.
Vanadium
Vanadium was discovered in 1801 and has been used since the beginning of the 20th century. for alloying cast iron and steel. It increases hardness, elasticity, wear resistance and tear resistance. Titanium-vanadium alloys are used for the manufacture of jet aircraft and space technology. Alloys of V with Cu, Ta, Nb, Zr, Ni, Co, Al and Mg are also known. In the chemical industry, vanadium is used as a catalyst in oil cracking and in the production of paints and rubber.
Nickel
Nickel has been known since ancient times, but industrial production began in the first half of the 19th century. Nickel is used to coat metal products to give them high chemical and thermal resistance. Additive to steels increases their toughness, elasticity, and anti-corrosion properties. Alloys of Ni with Cu, Zn, Al, Cr are also used; the coin alloy contains 75% Cu + 25% Ni.
Cobalt
Cobalt paints have been used since ancient times. Metallic cobalt was first obtained in 1735. A sharp increase in cobalt consumption dates back to the beginning of the 20th century. Currently, over 40% Co is used for the production of alloys and super alloys, superhard alloys of Co with Ni, Fe, Cr, W, Mo.
Molybdenum
Molybdenum was discovered in 1778, but it found widespread industrial use only in the 20th century. Over 80% of all mined molybdenum is used in the metallurgical industry, mainly for alloying steels and producing super alloys. Molybdenum steels acquire high hardness, toughness, refractoriness, acid resistance and a number of other valuable properties. Metal molybdenum is used in the production of electric lamps and vacuum devices. In addition, it is used in chemical, oil refining, ceramics, glass and other industries.
Tungsten
Tungsten in the form of the WO3 compound was discovered in 1781, and its industrial use for alloying steels began at the end of the 19th century. Tungsten is used in the production of special steels; the addition of tungsten to steel increases its hardness, strength, and refractoriness; these are high-speed, tool, and armor steels used in the manufacture of weapons and projectiles. Tungsten in combination with Cr, Ni, Co is used for the production of heat-resistant and superhard alloys - pobedit, carbides, borides.
List of used literature
1. Peshkovsky L.M., Pereskokova T.M. Engineering Geology 1982
2. Kononov V.M., Krysenko A.M., Shvets V.M. Fundamentals of geology, hydrogeology and engineering geology, M. 1978.
3. Belevtsev Ya.N. Iron Belt of the Earth 1987
4. Krasulin V.S. Handbook of geological technician 1986
5. Tsytovich N.A. Soil mechanics 1983
6. Albomov M.N. Ore geology 1973
7. Aristov V.V. Search and exploration of mineral deposits 1989
3
Recommended pages:
Use the site search: