Copper production methods
Currently, several methods for producing copper have been developed. The main ones are:
- pyrometallurgy;
- hydrometallurgy;
- electrolysis.
The largest quantity is produced using the first method. With its help, almost 90% of all metal is obtained. It is quite labor-intensive and time-consuming. The technology for producing copper using this method includes several stages that enrich the incoming material and sequentially obtain the finished material. Each stage contains a strict sequence of technological tasks. Typically, a copper smelter performs the full range of operations.
To obtain the so-called copper cathode, a third method is used. This method is completely called electrolytic refining with subsequent deposition of the finished product on the surface of metal plates.
Fire refining technology for blister copper
This method of obtaining pure copper is used when the starting material is copper scrap.
The process takes place in special reverberatory furnaces, which are fired by coal or oil. The melted mass fills the bath, into which air is blown through iron pipes:
- pipe diameter – up to 19 mm;
- air pressure – up to 2.5 atm;
- oven capacity – up to 250 kg.
During the refining process, copper raw materials are oxidized, sulfur burns out, then metals. Oxides do not dissolve in liquid copper, but float to the surface. To remove them, quartz is used, which is placed in the bath before the refining process begins and is placed along the walls.
If the scrap metal contains nickel, arsenic or antimony, the technology becomes more complicated. The percentage of nickel in refined copper can only be reduced to 0.35%. But if other components are present (arsenic and antimony), then nickel “mica” is formed, which dissolves in copper and cannot be removed.
Stages of pyrometallurgical copper production
This method is effectively used for processing ore with different copper contents. It consists of the following sequence of actions:
- preparation (enrichment) of extracted raw materials;
- direct smelting onto matte;
- converting the resulting matte;
- final refining.
Each technological process is carried out using the necessary processing methods. To isolate blister copper, so-called purging is performed. Next, the copper is placed into molds or poured onto slabs. It remains contaminated with various impurities and does not have the properties of pure copper.
The essence of the process is to supply air under pressure through the liquid melt of copper matte. It is produced in special converters, which can be positioned vertically or horizontally. Subsequently, enriched copper ore concentrates are sent for final processing.
Enrichment
Initially, the copper content in the mined ore does not exceed six percent. To produce copper with the best efficiency, it is necessary to enrich the mined ore. This production is intended to obtain a concentrate that will contain more than 10% copper. In some cases it can be increased to 35%.
The main method of beneficiation of sulfide copper-nickel ores is flotation. To increase the efficiency of enrichment, a magnetic separation operation is first performed. It promotes the release of pyrrhotite into an independent concentrate. The possibility of carrying out magnetic separation is due to the relatively high magnetic susceptibility of pyrrhotite.
The process itself includes the following operations:
- preliminary crushing and subsequent grinding into small particles (this is carried out until grains of no more than 0.05÷0.5 mm are obtained);
- flotation enrichment, which is based on the processing of non-wettable ore particles together with bubbles of blown air when they rise upward in the form of foam (oil is added for the efficiency of the process), the waste rock, being wetted, falls down.
After receiving the enriched material, proceed to the next stage.
Burning
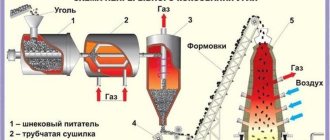
Pyrometallurgy defines two types of roasting process. The first is the so-called oxidative roasting. It produces partial oxidation of sulfides of copper concentrates. This process occurs in one of three modes: kinetic, diffusion and intermediate. Each of them is characterized by the rate of crystal chemical transformation and the value of the diffusion coefficient.
The correct choice of these parameters allows you to significantly reduce the sulfur content and obtain matte of the required concentration. This firing is carried out in special units. They are called kilns. With their help, it is possible to reduce the moisture content to five percent and at the same time reduce the sulfur content. The modern scheme of this process involves carrying it out in a fluidized bed or in a suspended state.
The second method involves heating to a temperature that activates the oxidation of sulfur sulfide. Higher fractions go through a stage of dissociation. The lower fractions are subject to oxidation only slightly.
The choice of optimal temperature for this process depends on the following conditions:
- fuel combustion process parameters;
- heat transfer characteristics;
- the quality of the insulating properties of the furnace (its durability of the lining);
- heat transfer characteristics of the processed material itself.
The most popular method is roasting copper concentrate in multi-hearth furnaces. They simultaneously carry out mechanical mixing of the loaded mixture. The greatest efficiency of the technological process is manifested in furnaces of a ten-pod design. In such furnaces, not only is sulfur removed most effectively, but the concentrate is also mixed efficiently with the added additives and fluxes. In this case, such a furnace plays the role of a mixing apparatus. The oven maintains a temperature between 450 and 500 degrees. The composition of the loaded mixture and the quality of firing (desulfurization) depend on the optimality of the selected parameters.
In addition to this method, there is firing of finished concentrates in a fluidized bed. To implement it, special units are used that are capable of creating such conditions. Their complex and expensive design significantly limits their use.
Melting for matte
The main components in the raw materials for producing matte are sulfides of two metals: iron and copper. It contains oxides of various metals, for example, aluminum, calcium. Carrying out the melting process allows you to obtain two products in liquid form. One is matte, in which copper is concentrated. It goes there from the oxides of the charge. The second is slag. It stores the remaining connections.
The raw material for smelting is the prepared concentrate. It is mixed with flux. They must stimulate this process. Such additives are limestone or quartz. Matte alloy is produced in several ways. For this purpose, reverberatory, shaft and electric arc furnaces are used.
The technological process of melting in reverberatory furnaces has gained the greatest popularity. They have the following geometric dimensions: up to forty meters long, width does not exceed ten meters and the maximum height from the bottom to the roof should be no more than four and a half meters. Under the stove, rests on an equipped foundation. It is made in several ways. Special silica bricks can be used, or they can be welded from quartz sand. The most optimal hearth thickness is considered to be from 0.6 meters to 1.5 meters. The walls are lined with magnesite-chromite bricks from the inside. The vault is made of an arched spacer-trapezoidal shape. To extract the finished matte, special holes are prepared. After the unloading operation is completed, they are closed with a clay plug. In some structures, special siphon devices are installed for unloading.
Refining using copper cathode
The refining process is designed to separate pure copper from various additives and impurities. In modern industry, it is considered economically feasible to carry out this process in two stages. The first is temperature refining, the second is electrolytic. The second method is carried out using copper cathode.
Electrolytic refining allows you to solve two problems:
- Deep cleaning from impurities.
- Ensuring high electrical conductivity.
Depending on the composition of the raw material, in some cases it is possible to obtain associated metals (silver, selenium and even gold). The technological process itself takes place in special baths up to 5 meters long and up to 1.5 meters deep. The walls of such baths are treated with acid-resistant materials. A mounting system is created above the bath, to which the cathodes are attached. Flat plates made of pure copper are used as cathodes. One plate acts as a cathode, the second as an anode. The bath is filled with electrolyte. The electrolyte used is sulfuric acid (H2SO4) in which copper sulfate (CuSO4) is dissolved. A low voltage of 0.4 V is applied to these cathodes. After the circuit is closed, the process of electrolytic dissolution of the anode begins. Under the influence of a potential difference, copper ions move from the anode to the cathode, settling on it in the form of pure copper. The electrolyte is periodically renewed. This is necessary, since metal solutions are formed in its composition, which slow down the electrolysis process. In addition, sediment called sludge accumulates at the bottom of the bath. It is also unloaded periodically. At modern enterprises, complete dissolution of the anode occurs within 30 days.
The unloading sequence is carried out at intervals of six to twelve days. The electrolysis process is quite electrically intensive. To obtain one ton of pure copper, it is necessary to provide a power of up to 350 kW.
The resulting cathodes are sent for further processing. As a result, individual ingots or blanks of a given shape are obtained. Melting of cathodes is carried out in reverberatory or shaft-type furnaces. The temperature at which the cathodes melt is created by burning natural gas, using electric arc or induction installations. The resulting copper is poured into ready-made molds. To obtain wire, it is placed in so-called wirebass. The entire process takes place in continuous or semi-continuous casting plants.
RAW MATERIALS FOR OBTAINING COPPER - COPPER ORES
To obtain copper, copper ores are used, as well as waste of copper and its alloys. The ores contain 1-6% copper. Copper, like many other non-ferrous metals, is becoming increasingly scarce. If in the 19th century. Copper was mined from ores containing 6-9% of this element, but now 5% copper ores are considered very rich, and the industry of many countries processes ores containing only 0.5% copper. In ores, copper is usually found in the form of sulfur compounds (copper pyrite or chalcopyrite CuFeS2, chalcocite Cu2S, coveline CuS), oxides (cuprite Cu2O, tenorite (CuO) or hydrocarbonates (malachite CuCO3 × Cu(OH2), azurite 2CuCO3 × Cu(OH) 2).The gangue rock consists of pyrite FeS, quartz SiO2, magnesium and calcium carbonates (MgCO3 and CaCO3), as well as various silicates containing Al2O3, CaO, MgO and iron oxides. Copper ores sometimes contain significant amounts of other metals: zinc , tin, nickel, gold, silver, silicon, etc. Copper ores are divided into sulfide, oxidized and mixed ores. Sulfide ores are usually of primary origin, and oxidized ores were formed as a result of the oxidation of metals in sulfide ores. So-called native ores are found in small quantities , in which copper is in free form.
Copper production in Russia and the world
According to analytical agencies, the Russian Federation confidently occupies fifth position among countries engaged in the mining and production of pure copper. Copper production in Russia on average per year is 860 thousand tons. The basis of the modern structure of copper production is made up of three large holdings: OJSC MMC Norilsk Nickel (Norilsk Nickel), LLC UGMKHolding (UMMC) and CJSC Russian Copper Company (RMK). These companies carry out a full production cycle from ore mining to the production of finished ingots, rolled products and wire. Each holding includes several enterprises equipped with the most advanced production technologies. Thanks to dynamic development, last year it was possible to increase copper production by seven percent.
Global copper production is fairly consolidated. Almost 35% of this metal is produced by the five largest companies. These include:
- Codelco (Chile).
- Freeport-McMoRan (USA).
- Glencore (Switzerland).
- BHP Billiton (Australia).
- Southern Copper (Mexico).
These companies obtain almost 80% of copper from primary raw materials (that is, they carry out a full processing cycle) and produce 20% as a result of processing incoming scrap. In Europe, the largest copper producers are: Poland, Portugal and Bulgaria. Each plant is capable of producing a wide range of copper products. Despite the current crisis, copper still remains a sought-after metal. One of the serious disadvantages inherent in this production is environmental problems. Assessments of emissions from copper smelters showed high levels of ambient air pollution. It contains a large number of chemical compounds harmful to health (cadmium, mercury, arsenic, lead, nitrogen oxides and carbon).
Factors of location of non-ferrous metallurgy in Russia
The location of non-ferrous metallurgy gravitates towards sources of raw materials, fuel and energy. Due to the low metal content in ores, their primary processing (i.e. enrichment) is strictly tied to the areas of raw material extraction. But the next stages of metallurgical processing are mainly focused on fuel bases (production of nickel and zinc) or cheap electricity (production of aluminum, magnesium, titanium). This, however, is only a “general scheme” of placement, and life, as we know, is much richer than any schemes.
Raw materials, fuel and energy have different effects on the location of different enterprises in the industry. Thus, the smelting of blister copper (which still contains impurities) occurs in raw material areas and areas rich in fuel. The final stage - copper refining (i.e. its purification) - can also be located in areas of consumption.
In the aluminum industry, at the first stage, a concentrate is produced from aluminum ores (bauxite or nepheline). It is called alumina and is pure aluminum oxide. At the second stage of production, aluminum is reduced from oxide at high temperatures in electric furnaces.
The production of alumina, being material-intensive, gravitates towards places where aluminum ores are mined, and the extremely energy-intensive smelting of aluminum metal gravitates towards cheap electricity.
If to produce 1 ton of steel at an electrometallurgical plant it is necessary to spend 9,000 kWh of electricity, then to smelt 1 ton of aluminum - already 18,000, and 1 ton of titanium - even 50,000 kWh!
Refining.
Copper grease: characteristics and application on a car
Almost all blister copper is refined sequentially by two methods - fire (pyrometallurgical) and electrolytic. Fire refining in reverberatory furnaces removes impurities Fe, Zn, Co, Ni and sulfur in the form of oxides from blister copper, and then dissolved gases, after which the copper is deoxidized. Previously, to remove dissolved gases from copper and restore Cu2O, raw wooden poles were immersed in the metal bath, the wood of which emits gaseous hydrocarbons that vigorously stir the melt. Now raw wood is being replaced by natural gas, oil-steam mixtures, and hydrocarbon by-products of other industries. After fire refining, the copper is fed to casting machines to cast anodes - square plates with ears for hanging in the electrolyzer. The anodes are placed in baths with an acidified copper sulfate solution. The cathode is a thin sheet of pure copper. During electrolysis, copper and other base metals (iron, zinc, lead and nickel) dissolve, leaving a slurry of silver, gold and platinum at the anode. The difference in electrical potential between copper and other basic impurity metals is large enough for copper in an acidic solution to be selectively deposited on the cathode with a purity of about 99.9%.
Metal extraction technology
To separate rocks that do not contain a valuable component, the flotation method is used. Only a small amount of raw materials containing copper in high concentrations is subjected to direct smelting. Metal smelting involves a complex process that includes the following operations:
- burning;
- fuse;
- conversion;
- fire and electrolytic refining.
Melting of raw materials.
During the roasting process of raw materials, the sulfides and impurities contained in it are converted into oxides (pyrite is converted into iron oxide). The gases released during roasting contain sulfur oxide and are used to produce acid.
Metal oxides formed as a result of the influence of a temperature gradient on the rock are separated in the form of slag during firing. The liquid product obtained from remelting is subjected to conversion.
Valuable components are extracted from blister copper and harmful impurities are removed by fire refining and other metals are removed by saturating the liquid mixture with oxygen and then pouring it into molds. Castings are used as an anode for the electrolytic method of purifying copper.
The raw material, which contains copper and nickel, is subjected to enrichment using a selective flotation scheme in order to obtain a metal concentrate. Iron-copper ores undergo magnetic separation.
Cuprous sandstones and shales, gangue and native metal ores are processed to extract copper concentrate. Enrichment is carried out using the gravitational method.
The flotation method is used for mixed and oxidized ores, but chemical methods and bacterial leaching are more often used.
High copper content is characteristic of concentrates extracted from chalcocite and bornite, and low copper content is characteristic of chalcopyrite.
Concentration of ore with low copper content can be carried out using a hydrometallurgical method, which consists of leaching copper with sulfuric acid. Copper and related metals, including precious ones, are separated from the resulting solution.
Application.
Copper has a unique combination of properties that have ensured its widespread use - high electrical and thermal conductivity, good corrosion resistance, high ductility and an attractive natural color. More than 70% of all copper consumed goes to electrical products, 15% to elements of building structures, 5% to parts of machines and mechanisms, 4% to transport structures and 4% to other types of products, including the manufacture of artillery weapons. . The construction industry consumes about 40% of all copper produced, electrical engineering and electronics about 26%, general engineering - about 14%, transport engineering - about 11%, consumer goods industry - the remaining 9%. Cables, electrical busbars, transformer windings and other electrical products are made from different types of copper. In cases where maximum electrical conductivity is required, “oxygen-free copper with high electrical conductivity” is used; in other cases, “technically pure” copper containing 0.02–0.04% oxygen is suitable. A small addition of arsenic increases the strength of red copper (a product of fire refining), but such copper, which contains oxygen, is difficult to weld. Copper with a reduced oxygen content has good casting properties and is used for the manufacture of chemical-technological equipment, copper pipes, automobile radiators, ship condensers, household water pipes, roofing material and other technical products.
Copper alloys are a group of common alloys whose properties vary widely. Some copper alloys containing cadmium, chromium, silver or tellurium have high strength with high processability and good electrical conductivity. The most famous and widely used copper alloys are brass (alloys with zinc) and bronze (alloys with tin).