- Reports and messages
- Geography
- Non-ferrous metals
Natural materials such as non-ferrous metals fit perfectly into human life.
They have become an indispensable part of absolutely any production, especially where technology is used. Metal as a material is quite simple and has its own characteristics and characteristics. Its main features are thermal conductivity, malleability and electrical conductivity. Non-ferrous metals include not only the metals themselves, but also their alloys. These do not include only iron. These materials can be classified according to several principles. Among them there are noble, light, and refractory. In many countries, non-ferrous metals are still quite in demand. Their extraction depends on their location in the bowels of the earth. The extraction process may also differ. The extraction of each mineral and metal should be approached in a special way. Aluminum is perhaps the most common of all non-ferrous metals available. It is very popular due to its high electrical conductivity and ductility. As for the mechanical properties, here they are quite low. Another non-ferrous metal that deserves attention is copper. This is the most common metal among all others. Copper has good thermal conductivity, ductility, and electrical conductivity. It forms excellent alloys with other metals. Alloys of this kind are well used in mechanical engineering. A metal such as zinc is widely used. At the most ordinary temperatures it is a fairly brittle material. When heated and the temperature increases, zinc is forged perfectly. A metal like zinc. Resists corrosion well. But if alkali or acid is applied to it, under their influence the metal will begin to collapse.
Modern technology improves the use and application of non-ferrous metal every day. These materials are always valued in aircraft manufacturing, the chemical industry, nuclear and rocket construction. Many materials are used to make parts, wires and even wires. They are extremely resistant to many external factors. To strengthen material made from non-ferrous metal, people learned to coat it with special varnishes and paints. This gives the ability to last longer than he could. Copper makes excellent pipelines, vessels and containers. In aircraft manufacturing and modeling, alloys with enormous density and high properties are used. Non-ferrous metals remain strong and durable for a long time, which is why there is such interest in them. Their only drawback is that they are destroyed by exposure to oxygen. But people have learned to solve this problem too.
4th grade briefly
Location of industry enterprises [ edit | edit code ]
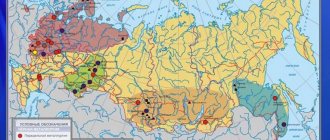
The location of non-ferrous metallurgy enterprises depends on many economic and natural conditions, especially on the raw material factor. In addition to raw materials, the fuel and energy factor plays a significant role.
Several main bases of non-ferrous metallurgy have been formed on the territory of Russia. Their differences in specialization are explained by the dissimilarity of the geography of light metals (aluminum, titanium-magnesium industry) and heavy metals (copper, lead-zinc, tin, nickel-cobalt industries).
Heavy metals [edit | edit code ]
Due to the low energy demand, the production of heavy non-ferrous metals is confined to the areas where raw materials are extracted.
- In terms of reserves, mining and enrichment of copper ores, as well as copper smelting, the leading place in Russia is occupied by the Ural economic region, on the territory of which the Krasnouralsk, Kirovgrad, Sredneuralsk, and Mednogorsk plants are distinguished.
- The lead-zinc industry as a whole gravitates towards areas where polymetallic ores are distributed. Such deposits include Sadonskoye (North Caucasus), Salairskoye (Western Siberia), Nerchinskoye (Eastern Siberia) and Dalnegorskoye (Far East).
- The center of the nickel-cobalt industry is the cities of Norilsk (Eastern Siberia) and Monchegorsk (Northern Economic Region), as well as the urban-type settlement of Nickel (Murmansk Region).
Light metals [edit | edit code ]
The production of light metals requires a large amount of energy. Therefore, the concentration of enterprises smelting light metals near sources of cheap energy is the most important principle for their location.
- The raw materials for aluminum production are bauxite from the Northwestern region (Boxitogorsk), the Urals (the city of Severouralsk), nephelines from the Kola Peninsula (Kirovsk) and the south of Siberia (Goryachegorsk). From this aluminum raw material, aluminum oxide is isolated in mining areas - alumina. Producing aluminum metal from it requires a lot of electricity. Therefore, aluminum smelters are built near large power plants, mainly hydroelectric power stations (Bratsk, Krasnoyarsk, etc.)
- The titanium-magnesium industry is located primarily in the Urals, both in areas of raw material extraction (Bereznikovsky titanium-magnesium plant) and in areas of cheap energy (Ust-Kamenogorsk titanium-magnesium plant). The final stage of titanium-magnesium metallurgy - processing of metals and their alloys - is most often located in areas where finished products are consumed.
ORES AND MINERALS OF NON-FERROUS METALS
ENRICHMENT PROCESSES
Characteristics of the main types of ores and non-ferrous minerals
metals
Minerals or mineral raw materials are natural mineral formations of the earth's crust of inorganic and organic origin, which, given the current state of engineering and technology, can be used with sufficient efficiency in the national economy in their natural form or after pre-processing.
According to their physical state, minerals extracted from the bowels of the earth are divided into solid (ore, coal, peat, non-metallic minerals), liquid (oil, mineral waters) and gaseous (natural combustible and inert gases).
The totality of minerals contained in the subsoil constitutes the concept of mineral resources, which are the basis for the development of such important industries as energy, ferrous and non-ferrous metallurgy, the chemical industry, and the production of building materials. Every year, up to 20 tons of rock mass are mined per person per year in the world. The doubling of the amount of mined rock mass occurs every 8-10 years. Of this rock mass, only 30-40% is used by industry.
Depending on the area of industrial application, mineral resources are divided into the following main groups: fuel and energy (oil, natural gas, fossil coal, oil shale, peat); ore , which are the raw material base for ferrous and non-ferrous metallurgy (iron and manganese ore, chromite, bauxite, copper, lead-zinc, nickel, molybdenum, tungsten, tin, ores of rare and precious metals; mining chemical raw materials ( phosphorites , apatites, table , potassium and magnesium salts, sulfur, barite, boron-containing ores, bromine- and iodine-containing solutions; natural building materials and non-metallic minerals , ornamental technical and precious stones (marble, granite, jasper, rock crystal, garnet, garnet, corundum, etc. ; hydromineral (underground fresh and mineralized waters). This classification of mineral resources is conditional, because the industrial use of the same minerals can be different, for example, oil and gas are not only energy fuels, but also raw materials for the chemical industry .
The development of the world economy is constantly accompanied by an increase in the consumption of fuel, energy and other types of mineral raw materials. Consumption of non-ferrous and alloying metals has increased 3-5 times over the past 100 years. In the 21st century, intensive growth in consumption of almost all types of mineral raw materials will continue. Only in the next 50 years, oil consumption will increase by 2-2.2 times, natural gas - by 3-3.2 times, iron ore - by 1.4-1.6, primary aluminum - by 1.5-2, copper - 1.5-1.7 times, nickel - 2.6-2.8 times, zinc - 1.2-1.4 times, other types of mineral raw materials - 2.2-3.5 times. In this regard, in the next 50 years the volume of mining operations will increase more than 5 times.
According to their material composition, metallic minerals are divided into ores of ferrous and non-ferrous metals. In turn, non-ferrous metal ores are ores containing heavy non-ferrous metals (copper, lead, zinc, nickel ), light non-ferrous metals (aluminum, magnesium), noble metals (gold, silver, platinum) and rare metal ores. The latter include ores containing light metals (lithium, beryllium, rubidium, cesium), refractory (titanium, zircon, hafnium, vanadium, niobium, tantalum, molybdenum, tungsten, rhenium), dispersed (gallium, indium, thallium, germanium, selenium , tellurium), rare earth (scandium, yttrium, lanthanum, lutetium, etc.) and radioactive (uranium, thorium, radium, polonium).
The main source of non-ferrous and rare metals are ores containing one or more non-ferrous, rare and noble metals in the form of natural minerals of a certain composition and crystalline structure, which are natural products of processes occurring in the earth's crust. Sometimes native metals such as gold, silver, platinum, and copper are found in ores. Industrial ores contain valuable elements in quantities at which their use is technically possible and economically feasible. Minerals that are extracted from the ore for further industrial use are called valuable or useful, and those that are not currently used are called host rock minerals . This division is conditional, because the same mineral in one case is considered a host rock mineral, and in another it is of industrial value and is extracted.
Read also: How to charge a car battery
The overwhelming majority of minerals are solids that obey all the laws of solid state physics. Less common are liquid ones, for example, native mercury. There are about 3,000 mineral species and about the same number of varieties in the earth's crust. Of the natural minerals, only 200-250 are of industrial importance; slightly more than 40 minerals are associated with the production of non-ferrous and rare metals. Each mineral is a natural compound with its own crystalline structure. Composition and crystal structure determine the physical and chemical properties of minerals, which determine their behavior in enrichment processes. There are crystalline minerals, amorphous ones - metalloids (for example, opals, limonite) and metamict minerals, which have the external form of crystals, but are in an amorphous glass-like state.
In nature, the most common minerals are the silicate class - about 25% of the total number of minerals; oxides and hydroxides - 12%, sulfides - 13%, phosphates and arsenates - 18% and others - 32%.
Based on the type of chemical bonds, minerals are divided into simple (native) and composite. In addition to the simple anions S 2-, 0 2-, 0H –, Cl – and others, complex salt-forming radicals [CO3] 2-, [SiO4] 4-, [PO4] 3-, etc. are often found in the structure. The basis of the modern classification minerals are based on differences in the type of chemical compounds and crystal lattices.
Minerals contained in ores of non-ferrous and rare metals can be divided primarily into sulfide and non-sulfide. Sulfide minerals include minerals that are natural compounds of metals and non-metals with sulfur. For example, the sulfide mineral galena PbS (lead luster) is the main lead mineral. It contains up to 86% lead. Chalcopyrite CuFeS 2 contains up to 34% copper, molybdenite MoS2 - up to 60% molybdenum.
Non-sulfide include oxides, silicates, aluminosilicates, carbonates, phosphates, etc. Oxides represent a significant part of non-ferrous and especially rare metal minerals, for example, cuprite Cu2O , containing up to 88% copper, ilmenite FeTiO3 , containing up to 52% titanium dioxide, rutile TiO2 , containing up to 95-100% titanium dioxide. The last two minerals are the main source of titanium. Tin is extracted mainly from cassiterite SnO2
, which contains up to 78% tin.
Silicate minerals are the largest group of minerals found in the earth's crust. In the upper mantle of the earth they make up up to 92%. These minerals include the bulk of the host rock minerals contained in the ores being processed, as well as a significant part of rare metal minerals (lithium, beryllium, etc.). Among the silicates, the most common are feldspars (on average 60%) and (quartz 12%), which is one of the main minerals of the host rocks of rare metal ores. It can be extracted into a separate concentrate and used in the production of glass and building materials. The copper silicate mineral, chrysocolla CuSiO3∙H2O, contains up to 31% copper. Zircon ZrSiO4 is the main mineral for obtaining zirconium compounds; it contains up to 67% zirconium dioxide and up to 16% hafnium dioxide.
Aluminosilicates minerals and host rock minerals. Lithium aluminosilicates (spodumene LiAl(SiO3)2 ) and beryllium (beryl 3BeO∙Al2O3∙6SiO2 ) are the main minerals for the production of lithium and beryllium. Spodumene contains up to 8% lithium oxide, and beryl contains up to 14% beryllium oxide.
Carbonates are a group of widespread minerals (more than 80 minerals) salts of carbonic acid. The most famous of them are calcite CaCO3, dolomite FeCO3 , malachite Cu2(CO3 ) (OH)2 , cerussite PbCO3 ,
smithsonite
ZnCO 3, etc.
Phosphates include minerals such as apatite F ,Cl) 2 , monazite ThPO4 .
Depending on the amount of valuable components in the ore, ores are divided into monometallic and polymetallic .
Only one valuable component is extracted from monometallic ores, for example, copper, tin, molybdenum, etc. Polymetallic ores contain two or more valuable metals (copper and zinc, lead and zinc, copper and nickel, molybdenum and tungsten). In nature, polymetallic ores are found much more often than monometallic ores. These ores, as a rule, are complex and even the extraction of associated metals from them becomes economically feasible. For example, copper-zinc ores usually contain small amounts of gold, which is associated mainly with sulfide minerals - chalcopyrite and pyrite. Gold recovery from subsequent metallurgical processing of copper and pyrite concentrate can account for a significant portion of the profit received from processing these ores.
According to the mineral composition of the main metal-containing minerals, ores are divided into sulfide, mixed and oxidized
.
Thus, in sulfide copper ores, the copper content in the form of sulfide minerals is at least 75%, in mixed ores - about 50%, and in oxidized ores by sulfide minerals, copper is represented by no more than 10-25%.
Based on the metal content, ores are distinguished between rich, poor and off-balance ores. However, this classification of ores is extremely conditional and depends on the state of the enrichment technology and the type of useful mineral. So, with the same metal content, for example, 0.1% molybdenum ore can be considered rich, and copper ore can be considered very poor. In off-balance ores, the content of the valuable component is so low that its extraction is unprofitable and such ores are classified as non-industrial.
There are also disseminated and continuous . In disseminated ores, crystals and grains of valuable minerals are dispersed in the mass of minerals in the host rock. Solid ores consist mainly of valuable minerals and contain small amounts of host rock minerals. For example, solid or pyrite copper-zinc ores contain more than 35% pyrite sulfur (sometimes up to 90 percent or more) and copper and zinc sulfides are distributed throughout the mass of pyrite, which in this case acts as host rock. In disseminated ores, the content of sulfide minerals, including pyrite, is 20-35% and they are dispersed in the mass of minerals in the host rocks.
Read also: How to dissolve super glue from hands
According to the size of grains of valuable minerals, ores can be very coarsely disseminated (more than 4-20 mm), coarsely disseminated (2-4 mm), finely disseminated (0.2-2 mm), finely disseminated (less than 0.2 mm ) and with very fine inclusions (less than 0.02 mm). The size of the inclusion of valuable minerals determines the required degree of ore grinding before beneficiation.
The mineral composition of ores is usually very diverse and complex both in material composition and in the size of minerals. From ores with large disseminations, it is quite easy to extract valuable minerals into rich concentrates, but from ores with very fine disseminations, this is very difficult to do using only enrichment methods. Such ores require, first of all, very fine grinding in order to open and free valuable minerals from intergrowths with minerals of the host rock or from each other. In this case, before beneficiation of some primary ores with fine and uneven dissemination of valuable minerals, it is necessary to crush and grind the ore to a particle size of less than 0.1 mm. The beneficiation of such fine ore is usually carried out using not only combined beneficiation methods, but also using metallurgical methods.
According to the conditions of formation, industrial types of ores are also classified into bedrock and placer
.
Bedrock ores occur at the site of initial formation and are located within the general rock mass.
In such ores, valuable minerals and minerals of the host rocks are in close association with each other. These ores, after being extracted from a mine or open pit, are crushed and ground before beneficiation. Placers are secondary deposits formed as a result of the destruction of primary bedrock ores under the influence of physical and chemical weathering processes. Under the influence of physical processes (temperature fluctuations, the wedging effect of water, ice, mineral salts, wind, glaciers, sea surf, etc.), mechanical destruction of the rock mass occurs. These processes are, as it were, preparatory to chemical weathering processes, which are carried out with the participation of oxygen, carbon dioxide, water, and microorganisms. In this case, sulfide minerals are oxidized and leached, and alkali and alkaline earth elements are removed. Placers are transported over long distances by river water flows and under the influence of sea waves. Chemically resistant minerals take on a rounded shape and are freed from intergrowths with other minerals. Therefore, the sands of placer deposits are not subject to crushing and grinding; gravitational enrichment processes are much simpler and cheaper.
In recent years, along with ores and placers mined from various deposits, sources of non-ferrous and rare metals have become the so-called technogenic raw materials - waste from mineral processing and waste from metallurgical production, for example, tailings from enrichment factories, rock dumps, slag from copper metallurgical production, etc. P.
Date added: 2015-06-10; ; ORDER A WORK WRITING
Non-ferrous is a group of different metals and their alloys.
Let's take a closer look at what non-ferrous metal scrap is.
There are two groups of metals:
Iron and its alloys are called black
The rest are non-ferrous or non-ferrous.
Their list is diverse:
are in demand today both in production and in scientific activities . Their areas of application are varied.
Scrap metal collection points are happy to buy non-ferrous metal scrap at competitive prices, and in order to avoid getting into trouble when handing it over, you need to be familiar with the types and know the standard classification of non-ferrous metals.
Development of ferrous metallurgy in the world
Ferrous metallurgy involves the following stages:
- development of ore deposits;
- raw materials preparation;
- smelting of materials such as cast iron, steel, rolled products and ferroalloys.
Stages of ferrous metallurgy
Ferrous metallurgy uses ore containing metals such as iron, chromium and manganese as the basic feedstock. In addition, many enterprises work with recyclable materials, such as depreciation scrap and metallurgical waste.
The use of scrap can significantly reduce the cost of production, since the stage of casting iron is skipped and the process of producing steel begins immediately. Coking coal is used as fuel for iron production.
Full cycle ferrous metallurgy produces its own product at each stage. Depending on this, there are several types of enterprises, such as:
- small capacity plants;
- metallurgy enterprises;
- full cycle plants;
- enterprises for the production of ferroalloys;
- electric steel plants.
In accordance with the location of coal or iron ore basins, ferrous metallurgy was often inconsistent in its localization. In the current period of scientific and technological breakthrough, the guidelines have shifted towards fuel and raw materials cargo flows.
Thanks to this, the size of enterprises under construction is reduced, and their geographical location does not depend on the deposits. For example, metallurgical plants in Western European countries and Japan are now located in areas with access to a seaport.
Classification of non-ferrous metals according to GOST
The current GOST 1639-2009 clearly indicates what belongs to non-ferrous metal scrap.
The classification of scrap is divided into four main sections that characterize it:
- Name;
- physical parameters;
- chemical composition;
- quality.
GOST metals and their alloys.
The section displays 13 types that are accepted in organizations for receiving recyclable materials.
Below is a table in which you can see a list of non-ferrous metals in one list and the number of individual types of scrap:
| Metal | Types of scrap |
| Aluminum | 32 |
| Tungsten | 17 |
| Cadmium | 2 |
| Cobalt | 3 |
| Magnesium | 8 |
| Copper | 13 |
| Brass | 23 |
| Bronze | 15 |
| Molybdenum | 9 |
| Lead | 11 |
| Mercury | 6 |
| Tin | 10 |
| Nickel | 26 |
Pure metal can rarely be found , since most scrap is made up of alloys.
Upon acceptance, belonging to one or another type is assessed by the element that is greater in percentage terms in recyclable materials.
This ratio can be determined using special equipment.
Non-ferrous metal scrap is divided into types according to the following criteria :
- origin;
- chemical composition;
- physical state.
The origin of the scrap may be as follows:
- industrial waste;
- marriage;
- substandard;
- scrap of finished products.
The chemical composition of non-ferrous metal scrap, which is determined in the laboratory, shows which metal or alloy it belongs to.
The most valuable recyclable materials are unalloyed metals with a low content of impurities. Physical parameters are just as important when passing as chemical ones.
According to these characteristics, scrap is divided into the following classes :
- A – directly refers to scrap and lump waste;
- B – includes shavings, tangled wire and small pieces;
- B - powdered waste (mainly found only in rare metals: tungsten, cobalt, molybdenum and titanium);
- G - other recyclables.
Leading countries in the production of non-ferrous metals
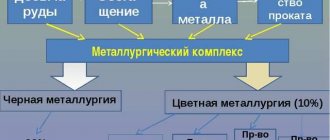
Non-ferrous metallurgy is divided into two large industries: heavy (production of Pb, Cu, Zn, Sn, Ni) and light (production of Al, Mg, Co, Ti, Li, Be and other metals). In terms of production scale, the metallurgy of ferrous metals exceeds non-ferrous metals by approximately 20 times.
Typically, non-ferrous metallurgy plants are built depending on the location of the deposits, since the amount of valuable metal in the ore is insignificant.
Other factors influencing the localization of non-ferrous metallurgy plants:
- energy;
- consumer;
- transport.
The initial production stages of copper, involving the extraction and beneficiation of ore, are localized in third world countries. Countries that do not have large copper reserves are focused on the final products of the production cycle.
The leading countries in copper production are occupied by the following countries:
- Chile;
- USA;
- Indonesia;
- Canada;
- Russia.
Transporting aluminum ore is more cost-effective than heavy metal raw materials, since the aluminum content of bauxite is about 50%. The total distance of bauxite transportation by sea is more than 7 thousand km, since their main deposits are located in regions with tropical and subtropical climates.
Countries producing the largest volumes of aluminum ore:
- Australia;
- Guinea;
- Jamaica;
- Brazil.
In aluminum production, the leadership is occupied by the USA, Germany, Japan, Norway, Russia, and Canada.
Safety
All non-ferrous scrap must be checked for:
- presence of radiation and harmful chemical contamination;
- explosion hazard.
When transporting scrap metal, it must be accompanied by documentation on radiation and explosion safety.
The concentration of harmful substances must not exceed the values specified in GOST 12.1.005.
The Russian Ministry of Natural Resources has identified five classes of chemical, radiation and explosion hazards of non-ferrous metal scrap:
- Hazardous waste with great harm to the ecosystem. These include mercury, polonium and plutonium.
- Highly hazardous waste, the consequences of which take nature thirty years to remove. These are alloys of lead, cobalt and molybdenum.
- Moderate danger , in which it takes ten years to restore the ecology. This is scrap mixed with copper, nickel, iron, zinc, aluminum and silver.
- Low hazardous waste, removal of the consequences takes three years. This includes scrap bronze.
- Low danger , such scrap does not harm the environment. This is the most common class among colored scrap.
Read also: Incandescent lamp on the diagram
Due to the expected harm to humans and nature, all operations with non-ferrous scrap require a license from the points accepting secondary non-ferrous metals. Checking for all types of hazards is carried out according to the following scheme:
Marking
According to GOST, all transported scrap must be marked with the following indication:
- names;
- GOST designations;
- designations of the type of recyclable materials;
- alloy grades.
Marking of non-ferrous metals and alloys must be firmly attached to the cargo during transportation and storage.
To determine the grade of metal, you need to look at the stamp book , a special document with all the markings of the metal or alloy you are interested in.
The large number of non-ferrous metals and various characteristics required their classification into separate types.
industrial systematization is in use , reflecting the historically established components of the metallurgical industry and the science of the same name.
The name itself does not fully reflect the essence of non-ferrous metal.
Only gold and copper are colored, while the rest are the usual grey-black shades.
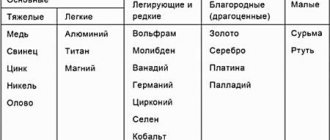
Science usually distinguishes the following types of non-ferrous metals and alloys:
- lungs;
- heavy;
- noble;
- refractory;
- scattered;
- rare earth;
- radioactive.
industry in Russia today is on the rise and includes:
- metal mining;
- ore beneficiation;
- metal smelting
Basic non-ferrous metals
The main non-ferrous metals include:
Aluminum is an excellent electrical conductor. It is flexible, which is both its advantage and disadvantage.
To give it strength add :
Such alloys are used for the production of :
- airplanes;
- sea and river ships;
- space shuttles;
- in construction;
- in the food industry.
Aluminum and its alloys are the cheapest type of non-ferrous metal scrap.
You can find it in a variety of household items, including:
Copper is a commonly found non-ferrous metal.
It also has good characteristics:
- plastic;
- good electrical conductor;
- good heat conductor.
It is in great demand in alloys and is used in various economic sectors.
Its alloy with zinc and tin is known - brass.
It can be found in:
find copper for scrap metal in:
- power cables;
- water pipes;
- household products.
Copper is highly valued at recycling centers.
Rare
Rare earth metals are used to improve the qualities of other metals and became widely used with the development of industrial production in the 20th century.
These are the following metals:
The name itself suggests that there is very little of these non-ferrous metals in the earth's crust. Also, previously, refractory oxides that form rare non-ferrous metals were called “earths” . They are extracted from oxides.
Today, rare earth metals can be found in all digital devices:
- smartphones;
- players;
- computers;
- in hybrid engines;
- in other electronics.
Alloys made from them have high characteristics , for example:
- anti-corrosion;
- strength;
- heat resistant.
Heavy
Let's consider heavy non-ferrous metals, collecting them in several lists.
The heaviest non-ferrous metals on Earth:
Rarely found in soil , it is generally the most expensive non-ferrous metal.
Also included in this group are:
All of them
have a high density and, accordingly, a lot of weight, which is why they get the name – heavy.
Lead is widely known and used in many industries , contained in:
It is made from:
Lead is also used to create protective aprons from radiation .
Has the following characteristics:
- low thermal conductivity;
- plastic;
- toxicity.
Therefore, lead must be used carefully, following all safety regulations.
Tin used to be called an alloy of lead and silver.
Today, tin is used in the metallurgical industry and the production of various alloys, which include:
- bearings;
- packaging foil;
- bronze;
- food tin;
- wires
Nickel is a heavy non-ferrous metal with high heat-resistant and anti-corrosion characteristics. Nickel is used in alloys. In stainless steel it is the main component.
Made from nickel :
- coins;
- armor;
- chemical equipment;
- wire;
- foil;
- a thread;
- powder;
- alkaline batteries.
in demand in:
Lungs
The definition of “light non-ferrous metals” includes metals with low density.
List of the most popular light non-ferrous metals:
The lightest non-ferrous metal is lithium. It is widely used in various alloys.
is used in:
- chemical industry;
- metallurgical industry;
- military-industrial complex;
- thermonuclear energy.
Lithium is also used in the manufacture of:
- optics;
- alkaline batteries;
- ceramic products.
The ductility of magnesium is not as good as that of copper and aluminum, which affects the welding properties of this metal. But it can be easily cut with a special tool. At the same time, the mechanical properties leave much to be desired. This it to be widely introduced into industrial production .