The significant chemical activity of metals (interaction with atmospheric oxygen, other non-metals, water, salt solutions, acids) leads to the fact that in the earth’s crust they are found mainly in the form of compounds: oxides, sulfides, sulfates, chlorides, carbonates, etc. In free form, there are metals located in the voltage series to the right of hydrogen (Ag, Hg, Pt, Au, Cu), although much more often copper and mercury can be found in nature in the form of compounds.
Minerals and ferrous rocks containing metals and their compounds, from which the isolation of pure metals is technically possible and economically feasible, are called ores
.
Obtaining metals from ores is the task of metallurgy.
Metallurgy
is both the science of industrial methods for obtaining metals from ores and a branch of industry.
Any metallurgical process is a process of reduction of metal ions using various reducing agents. Its essence can be expressed as follows:
M n+ + ne−→M
To implement this process, it is necessary to take into account the activity of the metal, select a reducing agent, consider technological feasibility, economic and environmental factors.
In accordance with this, there are the following methods for obtaining metals:
• pyrometallurgical;
• hydrometallurgical;
• electrometallurgical.
Pyrometallurgy
Pyrometallurgy is the reduction of metals from ores at high temperatures using carbon, carbon monoxide (II), hydrogen, metals - aluminum, magnesium.
For example, tin is recovered from cassiterite SnO2, and copper from cuprite Cu2O
calcination with coal (coke):
SnO2+ 2С = Sn + 2СО ↑; Cu2O + C = 2Cu+ CO ↑
Sulfide ores are pre-roasted in the presence of air, and then the resulting oxide is reduced with coal:
2ZnS + 302 = 2ZnО + 2SO2 ↑; ZnO + C = Zn + CO ↑ sphalerite (zinc blende)
Metals are also isolated from carbonate ores by calcination with coal, since carbonates decompose when heated, turning into oxides, and the latter are reduced by coal:
FeСO3 = FeО + СO2 ↑ ; FeO + C = Fe + CO ↑ siderite (spar iron ore)
By reduction with coal one can obtain Fe, Cu, Zn, Cd, Ge, Sn, Pb and other metals that do not form strong carbides (compounds with carbon).
Hydrogen or active metals can be used as a reducing agent:
1) MoO3 + 3H2 = Mo + 3H2O (hydrothermy)
The advantages of this method include obtaining very pure metal.
2) TiO2+ 2Mg = Ti + 2MgO (magnesium thermia)
3МnO2 + 4Аl = 3Мn + 2Аl2O3 (aluminothermy)
Aluminum is most often used in metallothermy, the heat of oxide formation
which is very high (2A1 + 1.5 O2 = Al2O3 + 1676 kJ/mol). The electrochemical voltage series of metals cannot be used to determine the possibility of reactions occurring in the reduction of metals from their oxides. The possibility of this process can be approximately determined by calculating the thermal effect of the reaction (Q), knowing the values of the heats of formation of oxides:
Q= Σ Q1 - Σ Q 2,
where Q1 is the heat of formation of the product, Q2 is the heat of formation of the starting substance.
Blast furnace process (iron production):
C + O2 = CO2, CO2 + C ↔ 2CO 3Fe2O3 + CO = 2(Fe2Fe32)O4+ CO2(Fe2Fe32)O4+ CO= 3FeO + CO2FeO + CO= Fe + CO2 (cast iron contains up to 6.67% carbon in the form of graphite grains and cementite Fe3C);
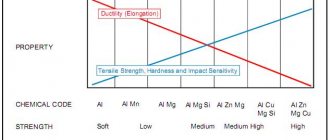
Steel smelting
(0.2-2.06% carbon) is carried out in special furnaces (converter, open-hearth, electric), which differ in the heating method.
Blowing air enriched with oxygen leads to the burning out of excess carbon, as well as sulfur, phosphorus and silicon in the form of oxides from the cast iron. In this case, the oxides are either captured in the form of waste gases (CO2, SO2) or are bound into an easily separated slag - a mixture of Ca3(PO4)2 and CaSiO3. To produce special steels, alloying additives of other metals are introduced into the furnace.
Leaching practice
In the first period of development of cyanidation, only percolation was used in wooden or round iron vats with a wooden false bottom made of beams laid in a cage. This lattice was covered with canvas, then with mats, matting or other rough durable material and secured with wooden planks. The capacity of the vats was 750–800 tons, their height rarely exceeded 3.5–4 m, due to the difficulty of infiltration of solutions; they occupied a large area.
Ore, crushed to 0.1–2 mm, was first loaded into the percolators manually, and later by machinery, and the thin silt part was preliminarily separated by classification and stored for subsequent processing in another way: it impeded the flow of solutions through the sands.
Later, with the development of leaching in pulps, sands (ephels) began to be leached in percolators, and silt in mixers.
In addition to the low intensity, as well as the difficulty of mechanizing loading and unloading, gold recovery in percolators was not high enough, it rarely exceeded 50–70% due to incomplete disclosure of gold particles by crushing and the density of the host rocks. It was necessary to use relatively concentrated cyanide solutions of 0.1–0.2% and abandon their continuous supply. Periodic flooding and subsequent drainage (descent) ensured the absorption of air into the pores of the rash and its oxygenation.
In modern gold hydrometallurgy, percolation has been preserved only in a few factories, and in most others it has been replaced by leaching in vats with stirrers.
The ore is crushed and ground in mills to sizes that ensure the opening of gold particles; the final size is determined experimentally. The w : t ratio during grinding is usually high, sometimes reaching 5, and excess water often has to be removed before leaching. This is done in thickeners, the performance of which is increased by adding flocculants and coagulants, in particular polyacrylamide and lime. After thickening, NS has w : t = 1–1.5, and sometimes with quartz ores it contains only 65% solid. Some plants do not require pre-thickening.
The pulp for leaching must have a weight : t corresponding to the composition and characteristics of the ore being processed. If the fineness of grinding is small - w : t = 0.8–1.2, with silt suspensions it reaches 3, and for some sulfide ores and concentrates 4–6. As pulp density increases, ore throughput increases, but leaching rates decrease due to increased viscosity, which inhibits diffusion.
Pachucas, vats with an impeller and diffuser, as well as various pneumomechanical mixers that combine mechanical and air mixing are used. The latter are the most productive, economical and provide good aeration.
Pachucas are cheap to work with and suitable for thick pulps; they have no moving parts, consume little energy, saturate the pulp with air, but require high rooms (the height of the vat reaches 14 m with a diameter of 4.5 m and a capacity of 156 m3) and are dangerous due to blockages in cases of unexpected cessation of blasting.
Tanks with a central airlift (Fig. 96, a) made of wood or iron have a useful volume of 55–250 m3 (diameter 4–8, height 4–6 m). In the center of the vat, a hollow vertical shaft rotates slowly (3–6 rpm), carrying a blade with rakes at the lower end, which are arranged like in thickeners - they move the sediment from the edges to the center. A thin iron tube is lowered into the shaft cavity from above, supplying compressed air. The airlift captures ore from the bottom of the vat with a flow of liquid and transforms it into a agitated state. The pulp spreads along two slightly inclined gutters mounted on a shaft with holes, through which it returns to the vat in thin streams.
Tanks with an impeller mixer and edge airlifts (Fig. 96, b) are the most productive due to turbulence and better oxygenation. The impeller makes up to 200 rpm, it creates intense circulation around the diffuser and in the volume of the vat. Edge airlifts provide good air supply, but the power consumption is approximately 1.5 times greater than with a paddle mixer. The volume of the vat is from 6 to 58 m3, its diameter and height are rarely more than 4.5 m. These mixers are beneficial for the periodic leaching of small quantities of rich concentrates.
Rice. 96. Pneumomechanical mixers: a – paddle with a central air lift; b – propeller with edge airlifts: 1 – full shaft; 2 – blade; 3 – inclined gutters; 4 – propeller (impeller); 5 – diffuser; 6 – airlifts feeding pulp from the bottom of the vat into the diffuser
Periodic and continuous is used .
According to the first method, the pulp is mixed with a strong solution of NaCN [from 0.03 to 0.1% (by weight) or up to 1 g/l] and filtered, the cake is washed on the filter.
Continuous leaching in 3–6 successive mixers (Fig. 97), in addition to less downtime, is easier to control and automate. The leached pulp is thickened in conventional thickeners or immediately filtered using vacuum filters.
Rice. 97. Scheme of continuous leaching of ore with a solution of sodium cyanide: 1 – thickener that separates excess water; 2 – sump for NS; 3 – sand pump; 4 – stirrer; 5 – intermediate sump; 6 – pressure tank; 7 – drum vacuum filter; 8 – filtrate collector
Mechanized and efficient drum and disk filters are not very suitable for washing. Because of this, many factories still use bulky frame vacuum filters, convenient for washing tailings with a small amount of water by counter-current wrapping the solutions.
For easily thickened pulps, continuous countercurrent decantation is sometimes used, often in multi-tier thickeners, in which the wash water and leaching tailings move countercurrently.
Rice. 98. Countercurrent decantation in multi-tier thickeners: a – leaching scheme with separation and washing of tailings by countercurrent decantation; b – multi-tier thickener: 1 – traps; 2 – transfer glasses; 3, 4 – tanks for washing solutions; 5 – pipelines
Hydrometallurgy
Hydrometallurgy is the reduction of metals from their salts in solution.
The process takes place in two stages: 1) the natural compound is dissolved in a suitable reagent to obtain a solution of a salt of this metal; 2) from the resulting solution, this metal is displaced by a more active one or reduced by electrolysis. For example, to obtain copper from ore containing copper oxide CuO, it is treated with dilute sulfuric acid:
CuO + H2SO4 = CuSO4 + H2
Copper is then either removed from the salt solution by electrolysis or displaced from the sulfate by iron:
СuSO4. + Fe = Cu + FeSO4
In this way, silver, zinc, molybdenum, gold, and uranium are obtained.
Cementation of gold and silver with zinc
Various researchers have repeatedly tried to isolate gold and silver from cyanide solutions using aluminum, sorption on activated carbon and other methods; however, so far only the concentration of solutions by ion exchange, which is discussed below, successfully competes with zinc cementation.
Precipitation by zinc occurs in the following sequence: Au, Ag, Cu.
It has been noted that pre-treatment with Pb(II) salts, which releases a loose layer of spongy lead on the zinc surface, accelerates cementation. Precipitation by lead is only possible for gold and silver, but not for copper. In this case, it itself produces insoluble Pb(OH)2, and the formation of plumbite is likely only at high alkalinity.
The rate of cementation is controlled by diffusion; it increases with the formation of a loose layer of spongy lead, which serves as a cathode base with a large surface area.
Preliminary removal of oxygen from the solution is necessary to economically consume zinc and increase the deposition rate. Another important condition is grinding the precipitant metal to increase its surface.
In the early days of hydrometallurgy, gold was precipitated with zinc turnings in box extractors, where the solution zigzagged through a series of filings filled with the turnings. At some enterprises this method has been preserved to this day, although it differs from the modern one in low productivity, high zinc consumption and worse sediment quality.
Now most factories use zinc dust (less than 0.1 mm) containing no more than 3% oxide. It is obtained by condensation of metal vapors. Before precipitation, solutions are clarified by filtering off the remainder of insoluble particles of different compositions and deoxygenated in a vacuum. Filter presses and often also frame vacuum filters are used for clarification.
Before being fed for precipitation, the solution is passed through a vacuum receiver - an iron cylindrical tank connected to a vacuum pump. The solution, through a hole in the lid, falls onto a nozzle made of wooden slats and, spreading over its large surface, quickly releases dissolved gases. The deoxygenated solution is collected in the lower conical part and released through a valve automatically connected to the supply regulator. The liquid level in the lower part of the apparatus is always the same; residual air pressure 3332–6665 Pa.
Installations for the precipitation of gold with zinc dust operate continuously. The clarified solution is sucked into a vacuum receiver for deoxygenation by a centrifugal pump, which, to avoid air leaks, is immersed in a tank with a cyanide solution. The same pump pumps it into the mixer, where zinc dust is continuously loaded with a belt or other feeder. The conical bottom of the mixer is connected by a pipe to the settling tank. Cementation occurs primarily during filtration.
Here, as with clarification, filter presses or vacuum filters with radial frames are used. In the latter, a propeller stirrer shaft runs along the axis of the vat, lifting sediment from the bottom. To mix the upper layers of the solution, a paddle wheel is mounted on the same shaft above. To avoid oxygenation, only smooth stirring of the pulp without forming a funnel is permissible (Fig. 99).
Fig.99. Installation for gold deposition with zinc dust: 1 – vacuum receiver for deoxygenation of cyanide solution; 2 – pump immersed in cyanide solution; 3 – mixer; 4 – frame vacuum filter
Two to three times a month, a set of frames is removed with a tap and the filter cloth is replaced on them, or the sediment is removed from it with a stream of water.
Filter presses , sometimes operating as part of such installations, are more expensive and more difficult to maintain, and they are used less frequently.
Before cementation, zinc dust is treated with lead acetate or lead nitrate. These salts in an amount of about 10% by weight of zinc are fed into the mixer or clarifier.
The completeness of precipitation of noble metals reaches 99.9% with a zinc dust consumption of 15–50 g/t of solution, depending on its concentration.
The composition of sediments is complex; in addition to 1–20% gold and 1–15% silver, they contain 4–20% lead, copper > 0.5%, as well as many other substances, including compounds of arsenic, antimony, selenium, tellurium, nickel and other elements, and in addition, an excess of metallic zinc, reaching 50% (by weight).
To remove foreign substances, sediments are treated by heating with a 10–15% solution of sulfuric acid in vats with stirrers and exhaust hoods. The latter prevent the possibility of poisoning people by the toxic gases AsH3, SbH3 and HCN released. After washing and drying, the sediment contains up to 20–50% gold, 30% silver and 4–7% zinc. In our country, complex centralized processing of sludge is practiced.
Electrometallurgy
Electrometallurgy
— reduction of metals in the process of electrolysis of solutions or melts of their compounds.
This method produces aluminum, alkali metals, and alkaline earth metals. In this case, melts of oxides, hydroxides or chlorides are subjected to electrolysis.
Examples:
a) NaCl (melt electrolysis) → 2Na + Cl2
b) CaCl2 (melt electrolysis) → Ca + Cl↑ c) 2Al2O3 (melt electrolysis) → 2Al + 3O2↑ d) 2Cr2(SO4) + 6H2O (electrolysis) → 4Cr↓ + 3O2↑ +6H2SO4 e) 2MnSO4 + 2H2O (electrolysis ) → 2Mn↓ + O2↑+2H2SO4 e) FeCl2 (electrolysis of solution) → Fe↓ + Cl2↑
Wrapping cyanide solutions
After the precipitation of noble metals, excess cyanide remains in the degold solution, which must be returned for leaching or neutralized and discarded. The latter is expensive and complex: cyanide and its complexes with copper and other metals are toxic even in small doses, in addition, small residues of noble metals in a large mass of solutions are of significant value.
The best option (the most complete wrapping) is limited by the “fatigue” of solutions, which, as cyanide complexes of copper, zinc, iron and other compounds accumulate in them, leach gold worse and worse, despite their reinforcement with fresh cyanide. Fatigue becomes noticeable already in the presence of 0.03% copper or 0.05% zinc.
The bulk of the solution after gold precipitation is returned for leaching; however, part of it in the form of poor washing solutions has to be dumped into a dump, otherwise it would not be possible to compensate for the constant flow of water for washing tailings and sediments. Wastewater carries with it small amounts of sodium cyanide, as well as ions Fe (CN)4-6, Cu (CN)(n-1)-n, Zn (CN)2-4, SCN–, CNO– and other compounds.
Rice. 100. Scheme of wastewater treatment of gold mining factories: 1 – tailings pond; 2 – mixers; 3 – settling tanks
The waste storage facility, which is an artificial pond, receives cyanidation tailings and degold solutions. From the upper clarified layer, the liquid is periodically drawn into a mixer, where it is treated with bleach. Cyanide and complex compounds containing it are oxidized to cyanate, which in turn is hydrolyzed:
ОN– + ОCl– = CNO + Сl,
CNO– + 2H2O = NH+4 + CO2-3.
Cyanidation using ion exchange resins
Many disadvantages of the cyanide process: low intensity, multi-stage nature, the need for large masses of toxic solutions and bulky large-sized equipment, constantly attract the attention of researchers seeking to choose other leaching reagents or change the processing of solutions. One of the successful solutions in this area turned out to be sorption leaching developed in our country, which is successfully used at some large gold recovery factories.
In this technology, the same reagents – cyanide and atmospheric oxygen – are retained for leaching; however, an ion exchange resin is also introduced into the pulp - an anion exchange resin, which simultaneously with leaching sorbs dissolved gold.
Ion exchange resins - cation exchangers and anion exchangers - solid organic polymers. Their chemical structures are characterized by spatial networks of hydrocarbon chains carrying ion-exchange groups. For cation exchangers, this is, for example, COOH or HSO3, which are capable of exchanging hydrogen for cations. In anion exchangers, amino groups of various substitutions add hydroxyl or another anion. Tetrasubstituted ammonium, bound to anions like the cations of strong electrolytes, produces strongly basic anion exchangers capable of ion exchange in acidic and basic media. Having designated the complex structure of the resin with the letter R and noting its solid phase with the top line, we write:
Like Au(CN)–2, other anions are also sorbed: Fe(CN)4-6, Cu(CN)3-4, OH–, Zn(CN)2-4. In a loaded resin, the exchange capacity of which (COE) is from 3 to 10 mg/eq/g, after sorption from a cyanide solution, for example, the following amounts of metals are found:
One of the possibilities for separating impurities is associated with selective sorption, the other with desorption. In this case, both the difference in the stability of complex ions and the strength of their bond with the resin are used. Scheme Fig. 101, which should be considered approximate, shows the sequence of desorption of impurities, and then noble metals, by various reagents. The last to be displaced is gold, which is simultaneously transferred from cyanide to thiourea complex:
Gold is precipitated from acidic solutions of thiourea by electrolysis, which is sometimes combined with elution, forcing the loaded resin to continuously pass through an electrolyte bath of a special device - electroelution. To avoid anodic oxidation of thiourea, graphite anodes are separated from titanium cathodes by anion or cation exchange membranes - thin films of appropriate resins. The former are impermeable to cations and neutral SC (NH2)2 molecules, while the latter retain anions and neutral molecules.
Rice. 101. Example of a scheme for sorption leaching of gold
For clarification, Fig. 101 it must be said that after the usual leaching of part of the gold in mixers, which proceeds more slowly than sorption, the pulp is fed into a chain of packs of a special device (Fig. 102), where the metal is further extracted from the ore and at the same time absorbed by the resin. Ion exchanger grains with a particle size of 0.4–1.6 mm are larger than ore particles crushed to 0.1 mm; they are separated on metal or plastic meshes installed in each vat. The resin is transferred to the previous one, and the pulp is transferred to the next pack, as shown in the diagram in Fig. 103.
Rice. 102. Circuit of devices for operation according to the diagram in Fig. 101: 1 – pulp; 2 – roar; 3 – pachuki for sorption leaching; 4 – vats for desorbent solutions; 5 – column for washing resin from ore particles; 6, 7 – columns for desorption of impurities; 8 – collection of electroelution solutions; 9. 10 – installations for electroelution and reprecipitation of gold by electrolysis; 11 – hydrogen cyanide absorbers; 12 – collection of alkaline solutions of sodium cyanide
The loaded resin is washed to remove trapped pulp and treated with a sulfuric acid solution to desorb zinc and nickel impurities.
Me (CN)n-2n+ nH+ = Me2+ + nHCN.
Rice. 103. Pachuk for sorption leaching: 1 – vat; 2 – airlift for mixing; 3 – airlift for transferring resin to the chain; 4 – the same, for supplying pulp to the separating mesh; 5 – dividing mesh
Up to 90% of gold and silver are deposited on the cathodes by electroelution for 6–8 hours. The copper impurity, most firmly bound to the resin, partially remains. Then it is additionally extracted along with iron and cobalt using an alkaline solution of ammonium nitrate, and the ion exchanger is returned to sorption leaching.