The English name of the mineral chalcopyrite is Chalcopyrite
The name chalcopyrite is derived from the Greek. chalcos - copper, pyros - fire.
Mineral synonyms: Copper pyrite , yellow copper ore , tovanite (Miller Brook, 1852), cupropyrite (Uzry, 1920). An altered chalcopyrite is gomichlin (Brighthaunt, 1858). Barnhardtite (barnhardtite; Ghent, 1855) is a mixture of chalcopyrite with other minerals.
Chalcopyrite and calcite
Crystallographic characteristics of chalcopyrite
The syngony is tetragonal; tetragonal scalenohedral c. With. L24L22P.
Two modifications: Tetragonal and cubic - high temperature. cubic X. is not homogeneous, but consists of thin lamellar and lattice intergrowths; cubic and tetragonal modification of X. habit: pseudotetrahedral, less often dipyramidal and scalenohedral.
Crystal structure
The structure of chalcopyrite is similar to the structure of sphalerite. If Cu and Fe are considered equivalent, then the chalcopyrite lattice will exactly correspond to the sphalerite lattice. The tetragonal nature of the lattice is the result of the alternation of Fe- and Cu-tetrahedra according to the law of a quadruple mirror-rotational axis; due to this alternation, the parameter along the c axis turns out to be doubled compared to sphalerite. Each S atom is surrounded by four metal atoms (2Cu + 2Fe) at the corners of an almost regular tetrahedron; Each metal atom is surrounded by 4S. Distances Cu - S 2.32, Fe - S 2.20. Cu and Fe apparently do not have fixed positions, and the composition of the cell corresponds to both Cu1+Fe3+S2 and Cu2+Fe2+S2.
Main forms:
Chalcopyrite crystals are mostly pseudotetrahedral or pseudooctahedral with an equal development of positive and negative tetragonal tetrahedra; also bipyramidal and scalenohedral. The most developed faces are usually p(112) with hatching || ribs (112): (101) and (112): (102) or matte, less common r(112) - smooth, shiny.
Properties of chalcopyrite
The main properties of the mineral include fragility (it can be scratched in everyday life by a hard object - for example, apartment keys). The oxidation process greatly reduces its decorative properties, and its magical properties are not fully understood. But first things first.
Physical properties
Physical properties include, first of all, its mechanical features (susceptibility to damage, fragility). Chalcopyrite has a very specific formula - CuFeS2. She gives him complete immunity to highly concentrated hydrochloric acid. However, the sulfide in the composition makes it completely soluble in sulfuric acid.
This feature allows the stone to be used as a raw material in the production of copper (although the iron must first be separated). With the help of sulfuric acid, the process of metal isolation is greatly simplified. Many articles have been written on the process of extracting copper from such ore.
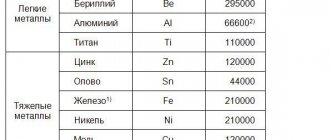
The hardness of the gem is low - from 3.5 to 4 units on the Mohs scale. The yellow color with a golden tint makes you think of expensive metal. Density ranges from 4.1 to 4.35 grams per cubic centimeter. There is no transparency as a species - the stone looks more like a metal nugget. The syngony of the mineral is tetragonal, the melting point is low for gems - only 1000 degrees Celsius.
The color of the unusual stone is plain copper, but often contains inclusions of more expensive metals - silver, gold. This is reflected in the appearance - natural “sparkles” appear on the surface of the specimen.
Chalcopyrite is a deceptive stone. It can be the basis for a more expensive ornamental material - malachite. But the oxidation of even a small nugget can take centuries.
Medicinal properties
The healing properties of the stone are wide. Thanks to the copper in its composition, it becomes beneficial for life and health.
The basic healing effects of the mineral include anti-inflammatory and antimicrobial effects - this is especially important during the season of illness.
The benefits for the digestive system are also noticeable. Nuggets are used to enhance activity in the digestive tract and provoke the release of acids to improve food digestion. It increases appetite and balances metabolism.
In neurology, the gem is useful in the treatment of common and intractable skin diseases. Among them are dermatitis, eczema, psoriasis. All of them do not have special medications to improve skin condition. Therefore, chalcopyrite, in terms of its healing properties, is unrivaled for these diseases.
The psychological help from “copper gold” is significant. For sleep disorders, especially in terms of annoying nightmares, the mineral provides serious support. A side effect is the treatment of stress and exhaustion.
A medical effect has been recorded - increased production of bile. Therefore, people suffering from stones in the bile ducts should limit their carrying of the stone.
Magic properties
The disdainful attitude towards the gem on the part of jewelers greatly slowed down the study of magical and astrological properties. It is not yet possible to assess the strength of this powerful mineral. It is known for sure that amulets made from it are quite powerful and have a strong protective nature.
The magnetic power of the stone is used by women to attract their betrothed. In the East, meditation practices with this mineral are common. A pleasant bonus is the effect of rejuvenation and improvement of overall health. It is believed that he is able to attract happiness into the home
Women's amulets and amulets made of copper pyrite give beauty, youth and protect against adversity. Their work is very much like a magnet - it attracts the pros and repels the cons.
Household amulets and chalcopyrite items are recommended for the most important premises. There will be no secrecy in such protection, but a strong impenetrable wall is ensured. The gem will repel troubles brought into the house by the elements (floods, storms, earthquakes) and people (theft, robbery, attacks). To ensure that the house is protected from negativity, gems are placed on all window sills in the house (on the right side).
In the field of trade, copper pyrite can bring good luck and success. To increase profits, they wear a neck amulet and carry it with them everywhere.
Form of being in nature
The appearance of crystals. Crystals are rare and are found only in drusy voids.
Mineral twins. Typically, chalcopyrite has twins {112}, {102}, {110},. Twins along (112) with fusion planes (112) or perpendicular to it are predominantly developed (twins of fusion and germination, pentads, polysynthetic twins); less commonly, (102) twins and complex intergrowth twins with a twin axis [001] and an intergrowth plane (110). Thin polysynthetic, often curved twins arise under dynamic influences. Parallel intergrowths with sphalerite and tetrahedrite are known (due to the similarity of structures), as well as almost parallel intergrowths with pyrite, cobaltine and galena. Often chalcopyrite grows on tetrahedrite, and sometimes tetrahedrite envelops it.
Chalcopyrite aggregates. Granular masses or individual grains (impregnations), sometimes crystals and their groups; Cryptocrystalline kidney-shaped sinter aggregates are relatively rare.
Photo of crystal intergrowths with galena
Chalcopyrite deposits
Stone deposits have been identified on almost all continents. True, it is impossible to find a pure mineral. It is found in other rocks, for example, fused with druses of quartz or chrysolites.
The properties of ore mined in different places differ:
- Russia. In the Urals, as well as the Kola Peninsula, large green minerals are found.
- Japan. The crystals are small, fused in the shape of pyramids.
- France . The most expensive specimens, similar to gold, are mined.
- Kazakhstan. The deposits are located near Dzhezkazgan. Small stones were found here.
- Mexico. In the Zacatecas region, small specimens are mined.
- South America. Chalcopyrites intergrown with sphalerite. Often they need to be separated.
Optical
- The color is brass-yellow, a unique shade typical of chalcopyrite, golden yellow.
- The mineral has a black streak with a greenish tint, and in places has a metallic shine.
- The shine is strong metallic.
- Low tide - often mottled tarnish
- Transparency Opaque
Mechanical
- Hardness 3-4.
- Density 4.1–4.3 (calc. 4.283).
- Cleavage according to (112) and (101) is imperfect.
- The fracture is conchoidal to uneven.
Quite fragile.
Industries of application of chalcopyrite
The ore is used in various fields:
- Industry. It is used for the production of copper, as well as the enrichment of copper-zinc ores. Mining of the mineral is accessible, it lies shallow, and there are a huge number of deposits. The process of extracting copper from it is considered profitable, although it consists of only a third of this metal.
- Collectible material. Nuggets with ribbed textured edges, multi-colored, variegated fragments and protrusions on the surface are especially valued.
- Jewelry making. The mineral is classified as an ornamental one. Jewelry with it is rarely made. As a rule, this work is carried out by single craftsmen. Large companies do not use it because of the low cost and low cost of the final product, as well as the complexity of processing. This is a fragile, brittle nugget that oxidizes and becomes covered with an unsightly film when exposed to oxygen.
Rings, earrings and pendants are made from chalcopyrite.
The frames for them are inexpensive metals (bronze, cupronickel), but sometimes silver is also used.
Chemical properties
Chalcopyrite dissolves in HNO3 with the release of S. The mineral does not dissolve in HCl. In polished sections, HNO3 and KCN are very weakly etched. The structural features of the aggregates are clearly revealed by etching with aqua regia vapor, concentrated HCl + CrO3 vapor, as well as a KMnO4+ KOH or HNO3+ KCl3 solution. In a closed tube there is sublimate S.
Other properties
Good conductor of electricity. Resistivity decreases significantly as temperature increases.
The melting point of the mineral is 1000°. Line expansion coefficient up to 40°, average 0.000017 (Niggli). On the differential heating curve in an atmosphere of nitrogen or carbon dioxide there is a sharp endotherm, a peak with a maximum at 560° and two insignificant endothermic deflections associated with the transition to other modifications. At high temperatures, a cubic variety is formed, isostructural with sphalerite. At temperatures above 250°, chalcopyrite retains CuFe2S3 (cubanite) in a solid solution; as the temperature decreases, the latter separates.
Artificial production of chalcopyrite.
Small crystals of chalcopyrite in the form of tetragonal tetrahedrons were obtained by the action of H2S on a weakly heated mixture of 2CuO + Fe2O3, as well as by treating a mixture of copper carbonate and Fe2 (SO4)3 with hydrogen sulfide water in a closed tube (according to Dölter), by the action of a weakly alkaline solution of CuCl2 on K.2Fe2S4 crystals (in the absence of air).
Diagnostic signs
Recognizable by its brass-yellow color, greenish-black streak, variegated tarnish, medium hardness (does not scratch glass).
Galena, chalcopyrus. and quartz. Polymineral aggregate
In solid masses, the mineral is similar to pyrite, but has a yellower color and less hardness and lacks cubic crystals. Unlike gold, it is fragile. It differs from cubanite and millerite in the shape of its crystals. Under a microscope, chalcopyrite is so characteristic that it is difficult to confuse it with other minerals, but in very small grains and in the absence of direct comparison with other sulfides it can be mistaken for cubanite, millerite, pyrite, or gold. Cubanite is creamier, more anisotropic; Millerite and pyrite also have a higher reflectivity, the first is harder and isotropic, the second is highly anisotropic and bi-reflective; gold is much brighter and has a peculiar shine when the nicols are crossed.
Satellites. Quartz, calcite, siderite, pyrite, galena, sphalerite, pyrrhotite, pentlandite, arsenopyrite, magnetite, cobaltine, tourmaline, enargite, hübnerite
How to spot a fake
Chalcopyrite is a popular imitation of gold. But they are also trying to falsify it. Under the guise of a mineral, they offer plastic or coated glass.
It is easy to distinguish one from the other:
- Scratch the sample. Even a fingernail can easily damage the coating on the imitation.
- Pass the sample over a hard surface. Chalcopyrite will leave a greenish-black mark.
- Look into the light. The natural mineral is opaque and impenetrable to light.
The mineral does not lend itself to elegant processing: mostly it is cabochons, beads or simple geometric shapes. Unlike plastic, chalcopyrite jewelry is rough. They look interesting though.
You can distinguish pyrite and chalcopyrite by color: the latter has a more intense yellow.
Origin and location
In nature, chalcopyrite can form under various conditions. It is found in various igneous skarn deposits in Cu-Ni, W-Mo-Sn, Pb-Zn and pyrite deposits, hydrothermal zones, and is also known as a mineral of some sedimentary rocks. As a companion of pyrrhotite, it is often found in magmatic deposits of copper-nickel sulfide ores in basic igneous rocks in association with pentlandite, magnetite, sometimes cubanite, etc. It is most widely developed in typical hydrothermal vein and metasomatic (including contact-metasomatic) deposits. It is commonly associated with pyrite, pyrrhotite, sphalerite, galena, fahlores and many other minerals. Nonmetallic minerals in these deposits include quartz, calcite, barite, silicates of various compositions, etc.
During exogenous processes, chalcopyrite is formed very rarely among sedimentary rocks under conditions of hydrogen sulfide fermentation during the decomposition of organic residues and the influx of copper-bearing solutions. Phenomena of its replacement of wood and organisms (along with chalcocite and marcasite) in phosphorite deposits were observed.
Cubic X. is found only in sulfide Cu-Ni deposits associated with ultramafic and mafic igneous rocks
During the weathering process, chalcopyrite breaks down chemically and produces copper and iron sulfates. Soluble copper sulfate, when reacting with CO2 or with carbonates in the presence of oxygen and water, forms malachite and azurite; with SiO2 hydrosols - chrysocolla; when interacting with various acids formed in the weathering zone, various salts are formed: arsenates, phosphates, vanadates, sometimes chlorides, etc. Under conditions of a very dry climate, various copper sulfates, easily soluble in seeping surface waters, are also preserved in the oxidation zone.
Pseudomorphoses of chalcopyrite, i.e. its replacement by secondary copper sulfides - bornite, chalcocite and covellite, are widespread in zones of secondary sulfide enrichment of copper deposits.
Chalcopyrite as a companion is found in varying quantities in almost all hydrothermal deposits of a wide variety of sulfide ores. In ores of many deposits it is an essential component and has independent industrial significance. In Russia and neighboring countries we have representatives of all genetic types of deposits in which chalcopyrite is the main copper mineral. In very rare cases, copper pyrite is formed in the zone of secondary sulfide enrichment of copper deposits.
In the Urals, so-called pyrite deposits are widespread, confined to strata of mostly metamorphosed effusive-sedimentary rocks of Paleozoic age. The main mineral (up to 60–80%) in the ores of these deposits is pyrite, with which chalcopyrite is paragenetically associated. Most of the Ural deposits belong to this type. In some deposits, chalcopyrite is closely associated with sphalerite.
History and origin
Chalcopyrite is one of the oldest minerals. Even before our era, it was found in the mining of tin and copper.
Shiny stones were often mistaken for gold (as was pyrite, which is similar in composition). Charlatans slipped it to gullible people under the guise of metal number one.
This effect was created by inclusions of gold, silver, and other metals contained in chalcopyrite.
It is not surprising that several names have been assigned to the mineral, and the most famous are fool's gold, false gold, donkey gold, and gold blende .
Chalcopyrite is the official mineralogical name of the mineral. At the everyday level, the term “copper pyrite” is used. It appeared three hundred years ago as a partial translation from ancient Greek: “chalcos” means “copper”.
The chemical formula plus the composition, in which copper and sulfur are almost equal, give grounds to classify chalcopyrite as copper-sulfur pyrite.
Place of Birth
In the highest temperature deposits associated with basic or ultrabasic rocks, it is found either in association with pyrrhotite and pentlandite - Monchetundra (Murmansk region), Sudbury (Canada), Ringerik (Norway), or in association with bornite in disseminated copper ores in gabbro - Volkovskoye field (Sverdlovsk region)
.
In contact-metasomatic deposits confined to the contacts of granitoids and carbonate rocks, chromium is observed among skarns (garnet, pyroxene-garnet); associated with pyrite, pyrrhotite, sometimes with arsenopyrite or with sphalerite and galena, magnetite, cobaltine; These are the Turinsky and Gumeshevskoye fields in the Sverdlovsk region, the Sayak group fields in Kazakhstan, Clifton-Morenci and Bisbee in the state. Arizona (USA), a number of deposits in Mexico, Japan and other countries.
The highest temperature vein deposits, in the ores of which copper pyrite is found, are some deposits of tungsten and molybdenum formations; These are, for example, the Karaoba, Eastern Kounrad, and Northern Kounrad deposits in Kazakhstan. In quartz veins of Cornwall (England), chalcopyrite is observed in association with cassiterite.
High-temperature deposits formed at shallow depths include the Braden deposit in Chile; in association with copper pyrite, tourmaline, quartz, pyrite, bornite, tennantite, molybdenite, siderite, rhodochrosite, anhydrite, and rarely galena, sphalerite, enargite, and hübnerite are observed here.
The group of medium-temperature deposits, in the ores of which chalcopyrite is observed in significant quantities, includes quartz-sulfide deposits of the vein type, deposits such as sulfide deposits, such as cuprous sandstones, as well as deposits of disseminated and disseminated veinlet copper ores.
Quartz-sulfide vein deposits rich in chalcopyrite include the Uspenskoye deposit in Kazakhstan, the Kafanskoye deposit in Armenia, and Butte in the state. Montana (USA).
In deposits such as pyrite deposits, chalcopyrite is observed mainly in association with pyrite, sphalerite, partly with fahlore, galena, and non-metallic ones - with quartz, barite, calcite; These are the deposits: them. III International, Levikha, Karpushinskoye and Degtyarskoye (Sverdlovsk region), Blyava and Yaman-Kasy (Orenburg region), Sibay (Russia), etc. in the Urals, Rio Tinto in Spain, Bor in Serbia. Lead-zinc and copper-zinc deposits, the ores of which contain chalcopyrite, along with galena, sphalerite, pyrite and other minerals, are numerous in Kazakhstan in the Rudny Altai (Belousovskoye, Zyryanovskoye, Ridderskoye, Zavodinskoye, etc.).
In deposits such as cuprous sandstones, chalcopyrite is the main mineral, but is usually closely associated with bornite, chalcocite, and fahlore (deposits of this type are classified by some researchers as hydrothermal, and by others as sedimentary). The largest representative of copper sandstone type deposits in the world is Dzhezkazgan (Kazakhstan); The richest deposits of the same type are found in the Belgian Congo (Katanga) and Northern Rhodesia.
In deposits of disseminated and disseminated veinlet copper ores associated with acidic igneous rocks, chalcopyrite is associated with pyrite, partly with enargite, fahlore, and molybdenite. The largest deposits of this type include Kounrad (Kazakhstan), Almalyk (Uzbekistan), and Bingham (Utah, USA).
The presence of copper pyrites in sediments of various ages has been noted in various areas. The formation of chalcopyrite in sedimentary rocks is associated with hydrogen sulfide fermentation; in some cases, organic residues are found along with it. Copper pyrite was discovered in the Devonian deposits of Tatarstan (in limestones, siltstones, mudstones) in association with pyrite, sphalerite, quartz in thin cracks and pores. The presence of chalcopyrite in Silurian phosphorites of Podolia (Ukraine) has been established.
Magic properties
The magical properties of chalcopyrite have not been fully studied by the occult community. But there are some results.
Overall Impact
The following spheres of influence of the mineral have been identified:
- In Europe, chalcopyrite stone is a protective amulet for a home, apartment or office. To do this, place a large agglomerate on all window sills on the right side: so that they cannot be seen by strangers. The magic of the stone will create the necessary aura, put a shield against external “blackness”: envy, anger, witchcraft.
- Europeans are confident that meditation on chalcopyrite is a powerful tool for women. They acquire a gentle character, visual attractiveness, and look younger. Those who want to get married or find a boyfriend are fulfilled in this regard.
- Chalcopyrite is a mineral that protects business people, especially managers and those involved in trade.
- Helps creative people attract inspiration.
Only natural minerals have magical powers.
Jewelry with copper pyrite
Talismans
Chalcopyrite has a stronger effect through jewelry:
- Bracelet. Worn on the main hand, it guarantees the financial success of business enterprises and creative projects. Or will attract the opposite sex.
- Ring. It will awaken inspiration and attract the attention of the chosen person.
- Brooch. To protect against negativity (ill-wishers, envious people, the evil eye) it is worn closer to the heart.
Any decoration with chalcopyrite “prompts” the owner to make the right decision, warning against spontaneous actions or rash words.
Practical use
The most common copper sulfide and the main copper mineral of most copper ores. Chalcopyrite-containing ores are one of the main sources of copper. Its industrial content in such ores usually ranges from 2–2.5%. Copper obtained at metallurgical plants is used both in pure form and in the form of alloys (brass, bronze, tombac, etc.). The main consumer of copper is the electrical industry. A significant amount of it is consumed in mechanical engineering, shipbuilding, the manufacture of equipment for the chemical industry, housing construction, etc.
Medicinal properties
Before using this mineral in powder form or as an ingredient in an ointment, you should consult your doctor. If he recommends the use of chalcopyrite, he will prescribe a suitable dosage. The dose should not be increased, as in this case an allergic reaction is possible.
Lithotherapists have discovered some healing properties of chalcopyrite:
- Anti-inflammatory, antimicrobial effect. A stone crushed to powder is added to a pharmaceutical ointment and lubricated on the skin for dermatitis, eczema, etc.
- Calming effect. For systematic insomnia, anxiety, depression, take orally a mixture of ingredients ground to powder: mineral, chalk, sugar.
- Stimulates the production of gastric juice, improves appetite.
Not everyone can use chalcopyrite for medicinal purposes, especially taking it orally, since copper, sulfur, and iron can cause problems, for example, increasing the production of bile. If you decide to be treated with this mineral, you should consult a doctor.
Chalcopyrite and zodiac signs
Beautiful ring with chalcopyrite
Chalcopyrite is loyal to all signs of the Zodiac:
- Capricorns will be protected from troubles and difficult life situations.
- Aquarians will learn with the help of the stone to look soberly at familiar things.
- Pisces will be able to fuel their strength on a physical and energetic level.
- Aries can use chalcopyrite as a talisman in their personal and family life.
- For Taurus, the stone will become a talisman in achieving success in career growth, business, and buying real estate.
- The mineral will teach Gemini how to find a common language with strangers, which will help expand their social circle.
- Cancers are recommended to use chalcopyrite for healing purposes.
- For Leos, the stone will add confidence to achieve strategic goals.
- Virgos will become softer towards loved ones.
- For Libra, the mineral will help get rid of suspiciousness and obsessions.
- With the help of the stone, Scorpios will be able to earn authority both in the work team and in a narrow family circle.
- Chalcopyrite will protect Sagittarius from mistakes in decision making.
The stone favors every person who wants to attract good luck in commerce, establish business connections and conclude the necessary contract. It will not bring a negative effect, but you should not overuse the properties of the stone. It is enough to take it with you to business meetings or corporate evenings several times a month to achieve the desired effect. Back to contents
Caring for products with Chalcopyrite
Products with Chalcopyrite are often purchased in specialized stores. But such jewelry requires special care and storage. Products made from this mineral should not be stored in the same box with other jewelry.
Also read: Pearls - a charming beauty from the depths of the water
It is better to put Chalcopyrite in special glass flasks, where it will be less susceptible to oxidation.
Since the mineral is very fragile and quickly becomes covered with an oxide film, caring for it involves regularly removing the film from the surface of the stone with a soft cloth.