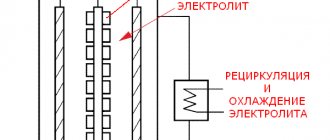
General information about anodic oxidation (anodizing) of aluminum.
The surface of aluminum and its alloys, due to their tendency to passivation, is constantly covered with a natural oxide film. The film thickness depends on the ambient temperature and is usually 2-5 nm. The corrosion and mechanical strength of aluminum can be increased tens and hundreds of times by subjecting it to electrochemical oxidation (anodizing). Examples of anodized parts are shown in Figure 1.
Anodization is the process of producing an oxide film from aluminum electrochemically from aqueous solutions. The density of such a coating is 2.9-3.8 g/cm3, depending on the production mode.
| Designation | An.Ox - anodizing without additional requirements; An.Oks.nv - filled with water; An.Ox.nhr - filled in chromate solutions; An.Ox. (dye color) - filled with dye, example - An.ox.ch; Anocolor - color anodizing obtained directly from the bath; An.Ox.tv - solid; An.Ox.iz - electrical insulating; An.Ox.emt - enamel; An.Ox.emt. (dye color) - enamel filled with dyes, example - An.ox.emt.ch; An.Ox.hr - anodizing from chromic acid electrolyte. |
| Coating thickness | 6-40 microns (for An.ox.tv the thickness is higher) |
| Microhardness (depending on the grade of aluminum alloy) | 1960-2450 MPa - D1, D16, V95. 2940-4900 MPa - A5, A7, A99, AD1, AMg2, AMg2s, AMg3, AMg5, AMg6, AMts. 4900 MPa - for enamel coating. |
| Electrical resistivity at 18°C | 1012 Ohm⋅m |
| Permissible operating temperature | 100o C (may increase when filling with dyes) |
Anodic oxide coatings are divided into the following groups:
• protective (9-40 microns) - requirements are imposed only on corrosion resistance;
• protective and decorative (9-40 microns) - not only corrosion resistance is important, but also appearance (this also includes colored and painted coatings);
• hard (usually >90 microns) - first of all, increased surface microhardness is needed. They can also perform the function of electrical insulation);
• electrical insulating (40-90 microns) - the magnitude of the breakdown voltage is estimated;
• thin-layer (up to 9-15 microns) - used, as a rule, for painting, or to maintain the gloss of the surface after coating;
• enamel.
• coatings with combined properties.
Figure 1 - Anodizing of metal. Examples.
An.ox.tv is a hard anodizing of aluminum, which differs from the standard An.Ox coating in its thickness and features of the application process. In some cases, the thickness of a hard coating reaches hundreds of micrometers, whereas in a conventional coating it is measured in tens. The high thickness and hardness of An.Ox.tv provides unsurpassed wear resistance of the aluminum surface.
The following are used as electrolytes:
• Low-aggressive phosphoric, citric, boric acid; • Aggressive sulfuric, sulfosalicylic acid, chromic anhydride.
Anodizing of metal always occurs at increased voltage, most often from 12 to 120 V. Sometimes the voltage can reach values that are enormous for electroplating - up to 600V.
The reaction products released at the anode can:
• completely dissolve (no coating is formed);
• create a tightly bonded thin (tens of nanometers) compact electrical insulating oxide coating on the metal surface;
• partially dissolve in the electrolyte and form a porous oxide coating tens and hundreds of micrometers thick.
After application, the porous coating can remain “as is,” compact in water, or fill. In the first case, the coating is perfect for applying paint and varnish materials and gluing. In the second, the coating retains its silver color and becomes more corrosion-resistant. In the third case, the coating can be given color without applying paints and varnishes. This is described in more detail in section 6.
Aluminum oxidation: anodic, chemical
, specializing in electroplating, offers its regular and new customers a wide range of services for nickel plating, phosphating, metal oxidation in various ways and much more. We will provide your metal structures with protection from adverse external influences and increase their service life.
You can find out a detailed description of the aluminum anodizing service on a special page. And then we will dwell in more detail on the theoretical part: the advantages and disadvantages of galvanic coatings.
As for products made from pure aluminum and its alloys, they are naturally resistant to corrosion. However, in industry, a higher degree of protection is often required. Therefore, there is a need to process the metal using chemical or electrochemical oxidation .
These types and their features will be discussed in more detail below. But, regardless of the chosen method, the applied coating must provide reliable protection of the part from the destructive effects of rust.
The chemical oxidation method is considered cost-effective and easy to implement. It is convenient for processing the inner surface of pipes, complex structures and large-sized products. However, the main disadvantage of chemical oxidation is that the protective layer is very thin, only 0.5 - 4 microns. It is not able to fully ensure corrosion resistance and long service life of the product. It is not advisable to use such hardware in aggressive environments. But, due to its high adhesion, the oxide film applied by the chemical method serves as a good basis for enamels and other paint and varnish coatings.
Depending on the chemical composition of the processed hardware and the solution applied to it, the protective effect and aesthetic appearance of the finished product can vary greatly. Optimal protection for aluminum during chemical oxidation is provided by a solution based on fluorides and chromic acid. The oxidation process of the part takes place at a temperature of about 100 degrees and lasts from 5 to 20 minutes. This treatment gives the metal a golden-yellow hue.
But, as already mentioned, films obtained in the process of chemical oxidation of aluminum do not have high protective and anti-corrosion properties. Their characteristics are inferior to the anodic (or electrochemical) coating. Therefore, aircraft, rocket, instrument making and other large industries use products made of anodized metal.
The process of anodic oxidation of aluminum is considered more labor-intensive and energy-consuming. It occurs when a current source is supplied (direct or alternating, sometimes combinations thereof). At the end, the finished products are covered with a durable film that provides resistance to corrosion. Such structures become suitable for long-term operation in aggressive environments.
In addition to strength characteristics, the method improves the appearance of aluminum. Decorative anodizing, which has recently become widespread, allows you to achieve a rich color palette. The color of the products varies from light yellow to brown.
Oxide coatings when anodizing aluminum are divided into porous and barrier. The first type provides good adhesion to the metal, the second gives the product high electrical resistance, which is important in the manufacture of capacitors.
Our managers will help you decide on the type of oxidation. Leave your request directly on the website or contact us by phone. We take on orders of varying complexity and are ready to complete any amount of work in the shortest possible time.
Composition and structure of aluminum oxide in the coating after coating.
Anodic coatings on aluminum can be thin non-porous and thick porous.
When obtaining thin coatings
in weak, low-aggressive electrolytes, an oxide is formed on the metal surface according to the reaction:
2Al + 3H2O – 6e → Al2O3 + 6H+
An illustration of the reaction is shown in Figure 2.
Figure 2 – Scheme of the formation of a thin oxide film in low-aggressive electrolytes.
All these solutions operate at high temperatures from 70 to 95º C, necessary to increase the electrical conductivity of the solution and reduce energy costs. And yet, the voltage on the bath remains very significant - 150-600 V. The duration of treatment is 15-30 minutes, and the thickness of the coatings does not exceed fractions of a micron. Due to their low porosity, thin anodic oxide coatings are poorly painted.
Thick porous anodic coatings
obtained from aggressive solutions (for example, from a solution of sulfuric acid). In coatings obtained from aggressive electrolytes, two layers are usually distinguished (Figure 3): 1) A thin, non-porous barrier layer adjacent to the metal (1), formed from the condition of 0.008 - 0.012 microns per 1 V of applied voltage, and usually amounting to 0.01 - 0.03 microns. 2) A thick porous layer (2), which is a system of cone-shaped pores penetrating the oxide film and has a thickness from several micrometers to millimeters.
Figure 3 - Structure of aluminum oxide layers obtained from aggressive electrolytes.
The structure of the thick porous anodic coating is confirmed by the results of electrochemical impedance spectroscopy (Figure 4).
Figure 4 - Bode plots for aluminum grade Al 6061, anodized in a sulfuric acid electrolyte followed by compaction in water when immersed in a 3.5% sodium chloride solution for a specified time. On the left is the Bode modulus, on the right is the Bode phase.
The Bode module graphs show the following areas:
• High impedance values at frequencies ≤ 1 Hz clearly indicate the characteristics of the barrier layer of the anodic coating.
• The quasi-horizontal region in the Bode modulus plot and the corresponding minimum region in the Bode phase plot characterize the resistance behavior of the porous layer. • The steep part at higher frequencies in the Bode modulus plot characterizes the capacitive behavior of the porous layer.
The equivalent electrical circuit of a porous anodic coating with compaction in water is shown in Figure 5.
Figure 5 - Equivalent electrical circuit of a porous anodic coating with compaction in water: Rsol - electrolyte resistance, Ro and Co - resistance and capacitance of the outer crystalline layer, Rpw and Cpw - resistance and capacitance of the pore wall, Rp and Cp - resistance and capacitance of the pore body, Rb and Cb are the resistance and capacitance of the barrier layer.
As for the composition of anodic-oxide coatings, thin non-porous films are mainly anhydrous aluminum oxide, which in its pure form is located at the boundary with the metal. In coatings of this type, from 0.6 to 20% boric anhydride (electrolytes with boric acid), as well as a significant amount of other ions, are introduced. A small portion of hydrated oxide Al2O3*H2O (boehmite) is found at the oxide-electrolyte interface.
Thick porous anodic oxide coatings consist mainly of amorphous aluminum oxide and partly γ-Al2O3. During the hydration of the oxide, due to the flow of electrolyte through the pores to their bottom, both physical adsorption of water and the formation of a boehmite phase Al2O3*H2O or bayerite Al2O3*3H2O can occur. The total water content in coatings obtained from sulfate electrolytes reaches 15%, while the barrier layer can contain up to 2% water. Hydration of the walls increases from the bottom to the mouth. Most researchers are inclined to believe that the water in the coating is not chemically bound, with the exception of the surface layers, where it is part of boehmite.
Thick coatings also contain a significant amount of electrolyte anions - up to 20%. For example, the mass fraction of sulfates can reach 14%. Electrolyte ions are distributed unevenly in the film: most of the sulfate ions are located in the surface layers of the oxide layer (up to 0.5 μm), throughout most of the porous layer the content of sulfate ions is constant (approximately 10%), and there are no electrolyte ions in the barrier layer . 50-60% of the anions are retained by capillary forces in the pores, the rest are firmly bound to the oxides and distributed fairly evenly throughout the thickness of the coating. The latter are called structural anions.
Metal impurities contained in aluminum alloys mostly remain in the oxide film (iron, copper, silicon, magnesium, calcium). Zinc and titanium are present in trace amounts at 0.1%.
In colored oxide films, inclusions of carbon, sulfur and their oxide compounds are found, which impart color.
Most ions are not removed from the coating either by prolonged washing with water at high temperatures or by the use of other solvents. Such a high bond strength of ions with the substance of the anodic film in the absence of simple stoichiometric relationships between the introduced ion and aluminum oxide indicates the introduction of ions into the elementary formations of the film. Apparently, some of the anions are retained by capillary forces in the pores of the coating, while the other part is chemically bonded to the walls of the porous layer.
With an increase in the amount of impurities in the metal, an increase in the temperature of the electrolyte and an increase in the anodic current density, the irregularity of the microstructure of oxide coatings increases - the perpendicularity of the growth of cells and pores is disrupted, their parameters become more uneven. The most chaotic structure is observed in films formed on aluminum alloys in solutions of chromic and orthophosphoric acids.
Figure 6 shows the original aluminum surface before anodizing.
Figure 6 - Initial aluminum surface before anodizing.
Figure 7 shows the surface of aluminum with oxide after anodization in a sulfuric acid electrolyte.
Figure 7 - Surface of aluminum with oxide, after anodizing in a sulfuric acid electrolyte.
As can be seen from Figures 4 and 5, after anodizing, microroughness caused by mechanical processing disappears on the aluminum surface. In this case, a dense porous oxide film is formed.
If you separate the porous and barrier layers, you can see the following picture (Figure 8):
Figure 8 - An example of an industrially anodized aluminum surface: a - replica of the porous layer, b - replica of the barrier layer, c - schematic image.
Our competence includes anodic oxidation of any type
Anodic oxidation can also be hard (in this case, the surface of the metal is affected not by one electrolyte, but by a combination of them) and color, in which it is important not only to strengthen the part, but also to change its color. In color anodizing there are such varieties as:
- adsorption coloring,
- electrolytic anodizing,
- interference staining.
TSK Industry+ LLC is ready to offer you any of the known types of aluminum anodizing, and at very affordable prices.
Theories of the formation of aluminum oxide films during anodization.
There are two theories of the formation and growth of anodic oxide coatings: structural-geometric and colloid-electrochemical.
3.1 Structural-geometric theory (Keller cells).
From the standpoint of this theory, when an anode voltage is applied to an aluminum electrode (i.e., connecting it to the “plus”), a compact oxide film (barrier layer 1-1.1 nm/V thick) is first formed, having a hexagonal cellular structure, and the growing coating will repeat it.
The outer part of the cells in aggressive electrolytes that dissolve the oxide begins to collapse in defective areas and turn into a porous coating. The destruction of the barrier layer, leading to the formation of a pore, occurs, according to some researchers, in the center of the cell, and according to others, at the junction of the cells.
Thus, under the influence of local effects of electrolyte ions, pores are generated in the barrier layer, the number of which is inversely proportional to the voltage. The diameter of the pores and their number depend on the nature of the electrolyte and the process mode. In the pore, the thickness of the barrier layer decreases and, as a result, the electric field strength increases, while the ion current density increases along with the oxidation rate. But, since the temperature in the pore channel also increases, which promotes etching of the pore, dynamic equilibrium sets in, and the thickness of the barrier layer remains practically unchanged. The cell size increases with increasing forming voltage. An example of a Keller cell is shown in Figure 9. The shape of the pore varies among different authors - from round to “star”.
Figure 9 - Keller cell.
The growth of the anodic-oxide layer occurs at the bottom of the formed pores due to the transformation of increasingly deeper layers of metal into oxide. Subsequently, under the influence of the electrolyte, the oxide forming the cell walls is hydrated. In this case, the adsorption of water, electrolyte anions and anodic reaction products occurs.
3.2 Colloid-electrochemical theory of Bogoyavlensky.
The presence of electrolyte anions in the oxide layer forced scientists to associate the growth and structural features of its structure with the colloidal structure.
From the perspective of Bogoyavlensky's theory (Figure 10), the formation of anodic oxide films begins with the appearance of monons - the smallest oxide particles with adsorbed electrolyte anions. The nucleation of monons occurs as a result of the meeting of ion flows. Monons are the seeds of future micelles.
With an increase in the number of monons, they turn into polyions - fibrous rod-shaped micelles of colloidal degree of dispersion, which form the skeleton of an oriented aluminum oxide gel. Electrolyte anions are introduced into it, partially losing their hydration shell.
The adsorption of anions and water, carried out through the intermicellar pores, causes a negative charge on the monomonium and micelles, causing them to be tightly pressed to the anode and fused with the metal, preventing the merging of the micelles into a pore-free layer. In this view, pores represent a natural intermicellar space.
Along with the processes of formation of micellar layers with the participation of anions, coupled processes of dissolution of the resulting oxide occur.
Figure 10 - Illustration of Bogoyavlensky's theory.
It is interesting to note that the sizes of Keller cells are close to the sizes of Al(OH)3 gel micelles. Interpretation of the mechanism of growth of the anodic film from the standpoint of colloidal chemistry makes it possible to explain the introduction of anions and cations of the electrolyte and individual components of the oxidized alloy into its structure. In this case, the coupling of the processes of oxide formation and its dissolution in the electrolyte is also taken into account by the colloidal theory.
3.3 Modern research into the structure of the coating.
Now it should be noted that the structure of anodized aluminum, in fact, can be very far from the ideal one described in theory. In particular, the theory speaks of regular hexagonal cells, in the center of which there is one pore. In fact, such a structure can only be obtained using special methods, for example, multi-stage anodization in certain modes. Examples of such “correct” coatings are shown in Figure 11. A more in-depth description of a nanostructured anodic oxide coating is beyond the scope of this article.
Figure 11 — Examples of ideal and near-ideal cells of a porous layer in an anodic oxide coating on aluminum.
More often you can see more “dirty” options. Examples of them were shown at the beginning of the article.
In addition, the theories do not imply the possibility of pore branching, which is observed in reality.
Oxidation. Oxidation process. Aluminum oxidation.
What is Oxidation?! Thermal oxidation.
We already know that some oxide films formed as a result of corrosion prevent further destruction of the metal. Metals that are easily subject to corrosion destruction: aluminum, magnesium and zinc are resistant to atmospheric conditions, since due to easy oxidation, a strong oxide film is formed on their surface. Taking advantage of these properties of oxide films, artificial oxide films are often created, this is called oxidation . It is widely used to protect firearms, machine tools, various devices, etc. With this coating, the parts acquire a characteristic black or blue color. Products made of iron, steel, aluminum and its alloys are usually subjected to oxidation.
There are two ways to oxidize steel parts:
— Wet ( chemical oxidation );
— Dry ( thermal oxidation ).
Chemical oxidation. Oxidation solution.
The most common chemical method for obtaining a protective film on products made from iron. Chemical oxidation is carried out in alkali solutions to which nitrate and nitrate salts of alkali metals are added.
For this purpose, solutions of the following composition are sometimes used: sodium hydroxide 800 g per 1 liter, sodium nitrate 50 g per 1 liter and sodium nitrate 200 g per 1 liter. The solution prepared in this way is heated to a boil and pre-treated (cleaned from dirt and grease) iron and steel products are immersed in it. After 20-30 minutes, the product acquires a beautiful black color with a bluish tint. Along with bluing, chemical bluing is also used. In this case, the product is immersed for 1-2 minutes in a mixture of molten salts, potassium nitrate and nitrate or sodium nitrate and nitrate, heated to a temperature of 310-350 ° C.
Features of aluminum oxide growth during anodization.
The formation of the oxide layer occurs at the bottom of the pores, where the only obstacle to the passage of electric current is a thin barrier layer, the thickness of which practically does not change during processing. From this point of view, it is possible to increase the thickness of the oxide layer without significantly increasing the voltage on the bath. The resulting pores have the shape of a cone, expanding towards the outside of the coating, since this part is exposed to the aggressive action of the electrolyte longer.
It should be noted that the formation of a porous structure is a necessary condition for the growth of the oxide layer. Aluminum oxide is a poor conductor of electricity, and the pores, although filled with electrolyte, have a very small diameter, so the anode resistance is many times higher than the cathode resistance and the electrolyte resistance. The change in the potentials of the electrodes themselves due to polarization is insignificant compared to the applied voltage, therefore the change in voltage over time at a constant current density is determined by the change in the ohmic resistance of the anode.
If the process is carried out at a constant current density, i.e. at a constant rate of oxide formation, the growth of the film will be inhibited by the increasing resistance of the electrolyte in the pores. Further growth requires either an increase in the applied voltage or etching of the pores. In practice, the second factor predominates. This is facilitated by the significant release of heat during anodic oxidation, with the bulk of the heat released in the barrier layer at the bottom of the pores. It is believed that during anodic oxidation in 15% sulfuric acid at 21°C and a current density of 1.29 A/dm2, the conditions created at the base of the pores correspond to a 53% sulfuric acid solution at a temperature close to boiling (about 128°C). In this case, the anode temperature increases by 10-20°, depending on the process conditions. Therefore, the growth of the oxide film at a constant current density is accompanied by a continuous increase in the rate of oxide dissolution. The maximum film thickness is reached when the rate of its formation under the influence of electric current becomes equal to the rate of chemical dissolution by the electrolyte. Excessive overheating of the electrolyte at the base of the pores and a local increase in its aggressiveness can lead to etching of the oxide layer and the production of low-quality coatings with increased porosity and poor adhesion to the metal.
The rate of dissolution of the oxide film in sulfuric acid increases with increasing temperature, while the activation energy of dissolution of the anodic film is estimated at 17 kcal/mol, which indicates that the rate is controlled by the kinetic stages of the chemical dissolution process. The rate of chemical dissolution of aluminum oxide is relatively high, especially in aggressive solutions of sulfuric acid.
Increased dissolution of the oxide is stimulated by an increase in the concentration of aggressive acid, temperature and duration of the process:
• at 20°C in a 20% solution of sulfuric acid for commercially pure aluminum, D16 and AL9 alloys, the dissolution rate is 0.2, 0.14 and 0.18 g/dm2∙h.
• when the electrolyte temperature doubles, the dissolution rate increases 6 - 7 times.
• when the concentration of sulfuric acid increases from 180 to 350 g/l, the dissolution rate increases by approximately 15%.
The dissolution of the oxide is expressed not only in the etching of the surface layer of the forming coating, but also in an increase in its porosity. The presence of copper and magnesium in aluminum alloys also slightly increases the rate of dissolution of the oxide in sulfuric acid.
Thus, the ratio of the rates of oxide formation and its chemical dissolution determines both the thickness and structure of the resulting anodic oxide coatings on aluminum.
Due to the fact that the resulting oxide layer has a high resistance, the electric current during the oxidation process is automatically redistributed to those areas where the resistance is lower. This creates conditions for obtaining an oxide layer of uniform thickness on parts of complex configurations. Therefore, the dissipative ability of electrolytes for the anodic oxidation of aluminum and its alloys is very high. However, it should be taken into account that if there is insufficient heat removal from the coating being formed, the possibility of local etching of individual sections of the coating arises, which will not be compensated by an increase in the current density in these areas. This will lead to local defects in the coating, up to its complete absence. Gradually, unsatisfactory conditions for coating formation can cover the entire part.
Due to the partial dissolution of the base metal during anodic oxidation, the current efficiency is always less than 100%. It decreases with increasing temperature and duration of electrolysis. For example, when oxidizing the D16 alloy in sulfuric acid at a temperature of 7°C, the current efficiency is practically independent of time and is 85%, but if the process is carried out at 20°C, then the current efficiency drops from 50-60% during the first 20 min to 15-30% with oxidation for 90 min. The electricity consumption for gas evolution is small and at moderate current densities (up to 1-2 A/dm2) does not exceed several percent, but can increase with increasing current density and the amount of alloying elements in the alloy being processed.
Chemical oxidation of aluminum and its alloys
EFHMO THOM Lecture 11
Chemical oxidation of aluminum and its alloys
Oxide coatings produced by electrochemical and chemical methods differ significantly in composition, structure and thickness. But there are general patterns in the mechanism of their formation. The dissolution of the film in both cases is the result of its interaction with the solution. During chemical oxidation in a solution of chromates, under their influence, a thin, non-porous film is formed on the surface. F– ions – into the solution.
or
SiF62–
. Activators break the continuity of the film, allowing the solution to penetrate to the surface and the growth of the oxide coating. The film growth rate during chemical oxidation is lower than during electrochemical oxidation, so the films obtained are an order of magnitude thinner.
The following electrolytes are used for the chemical oxidation of aluminum and its alloys.
1) Alkali-chromate. They form films with a thickness of no more than 2 microns and low mechanical strength. They are used as a primer for paint and varnish coatings.
2) Phosphate-chromate-fluoride. The thickness of the films formed in them is 3–4 microns, they have the best properties. Therefore, these films can be used as anti-corrosion coatings.
3) Chromate-fluoride. The films formed in them have low electrical resistance.
The color of the films depends on their thickness, the composition of the solution, and the alloying components of the alloy being processed. The inclusion of hexavalent chromium compounds gives a golden-yellow color, trivalent chromium - a greenish tint. A faint yellow color with a greenish tint is characteristic of thin films.
Compositions of the solutions used, g/l:
1) 40–60 Na2CO3
, 2–3
NaOH
, 10–20
Na2CrO3
.
Solution temperature 80–100° C
, treatment duration 5-20 minutes. Deviation from the optimal temperature deteriorates the quality of the coating.
2) 3–4 CrO3
, 3–4
Na2SiF6
.
Solution temperature 15–25° C
, treatment duration 5 minutes. When producing a solution, the temperature rises to 80°C, the processing time increases to 20 minutes.
3) 5–8 CrO3
, 40–50
Н3PO4
, 3–5
NaF
. As the solution is developed, the duration of treatment is increased from 5 to 20 minutes.
Poor quality coatings are removed by treating them for 5–10 minutes at 90–95° C
in a solution containing 150–180 g/l
CrO3
.
Oxide coatings of ferrous, non-ferrous and precious metals
Oxide coatings on steel
Oxidation of ferrous metals is called bluing. Chemical oxidation - alkaline and acid - has been used for a long time. Thicker and higher-quality coatings are obtained using the electrochemical method, but this method is less common compared to the chemical method.
During alkaline oxidation in hot solutions of sodium hydroxide (at 140–160 ° C
) on carbon and low-alloy steel, oxide films 1–3 microns thick, black, with a bluish tint, are formed;
on high-alloy steels - from dark gray to dark brown. They consist mainly of iron oxide Fe2O3
and admixtures of oxides of alloying components of the alloy being processed.
Acid oxidation is carried out in solutions of phosphoric acid or iron and zinc monophosphates with the addition of oxidizing agents - barium nitrates, potassium nitrates, manganese peroxide. It occupies an intermediate position between the processes of oxidation and phosphating. The resulting films reach a thickness of 5–6 microns and consist mainly of sparingly soluble phosphates. Their protective properties are better than those of films obtained by alkaline oxidation. The disadvantage of the process is the low stability of solutions compared to alkaline ones.
Before applying oxide-phosphate coatings, parts are activated in a 5–10% solution of phosphoric acid.
Regardless of the method of production, oxide and oxide-phosphate coatings, after washing, to improve their protective properties, are subjected to chemical treatment in chromate solutions, impregnation with mineral oil, inhibited lubricants, or hydrophobization.
Silver oxide coatings
Oxide or mixed oxide-salt films of dark brown or black color on silver are obtained by chemical or electrochemical treatment. In the first case, solutions based on liver sulfur became widespread. This preparation is obtained by fusing for 20–30 minutes a mixture of 2 parts by weight of sulfur and 2 parts of potassium carbonate K
2
CO
3
.
The resulting homogeneous alloy, after cooling, is crushed and dissolved in water.
For 100 parts of water - 2-3 parts of sulfur liver. The prepared solution must be used within 12 hours.
parts or coatings are processed in this solution for 2–3 minutes at a temperature of 60–70° C. Sulfur liver easily absorbs moisture, so the drug should be stored in a closed container.
For decorative finishing of silver products, you can use two-component solutions of the following compositions, g/l:
1) 5 liver sulfur, 10 ammonium carbonate (NH4)2CO3
;
2) 15 liver sulfur, 40 ammonium chloride NH4Cl
.
In these electrolytes, depending on the duration of treatment, films of light gray or dark blue color are formed.
Dark blue, almost black coatings are obtained by anodic treatment in electrolyte, g/l: 25-30 Na2S
, 15-20
Na2SO4
×10
H2O
, 5-10
H2SO4
. These components are added to water in the indicated sequence, after which 3-5 ml/l of acetone is added. Oxidation mode: anodic current density (0.1-0.5) A/dm2, temperature 18-25°C, duration 3-5 minutes.
Oxide coatings of intense black color, which are slightly more resistant to corrosion, can be obtained using alternating current with a density of (0.6-0.7) A/cm2 at a temperature of 60-80°C in an electrolyte containing 0.05 g/l permanganate potassium KMnO4
.
It is possible to form coatings of various colors on the surface of silver and its alloys, but they have poor mechanical resistance.
Phosphate coatings
EFHMO THOM Lecture 11
Chemical oxidation of aluminum and its alloys
Oxide coatings produced by electrochemical and chemical methods differ significantly in composition, structure and thickness. But there are general patterns in the mechanism of their formation. The dissolution of the film in both cases is the result of its interaction with the solution. During chemical oxidation in a solution of chromates, under their influence, a thin, non-porous film is formed on the surface. F– ions – into the solution.
or
SiF62–
. Activators break the continuity of the film, allowing the solution to penetrate to the surface and the growth of the oxide coating. The film growth rate during chemical oxidation is lower than during electrochemical oxidation, so the films obtained are an order of magnitude thinner.
The following electrolytes are used for the chemical oxidation of aluminum and its alloys.
1) Alkali-chromate. They form films with a thickness of no more than 2 microns and low mechanical strength. They are used as a primer for paint and varnish coatings.
2) Phosphate-chromate-fluoride. The thickness of the films formed in them is 3–4 microns, they have the best properties. Therefore, these films can be used as anti-corrosion coatings.
3) Chromate-fluoride. The films formed in them have low electrical resistance.
The color of the films depends on their thickness, the composition of the solution, and the alloying components of the alloy being processed. The inclusion of hexavalent chromium compounds gives a golden-yellow color, trivalent chromium - a greenish tint. A faint yellow color with a greenish tint is characteristic of thin films.
Compositions of the solutions used, g/l:
1) 40–60 Na2CO3
, 2–3
NaOH
, 10–20
Na2CrO3
.
Solution temperature 80–100° C
, treatment duration 5-20 minutes. Deviation from the optimal temperature deteriorates the quality of the coating.
2) 3–4 CrO3
, 3–4
Na2SiF6
.
Solution temperature 15–25° C
, treatment duration 5 minutes. When producing a solution, the temperature rises to 80°C, the processing time increases to 20 minutes.
3) 5–8 CrO3
, 40–50
Н3PO4
, 3–5
NaF
. As the solution is developed, the duration of treatment is increased from 5 to 20 minutes.
Poor quality coatings are removed by treating them for 5–10 minutes at 90–95° C
in a solution containing 150–180 g/l
CrO3
.
Properties of oxide coatings on anodized aluminum.
5.1 Corrosion resistance and porosity.
An anodic oxide coating on the surface of aluminum and its alloys has a beneficial effect on its corrosion resistance in many environments where the oxide is more resistant than the base metal. It successfully protects aluminum from atmospheric corrosion in neutral and slightly acidic solutions of inorganic salts:
• the resistance of anodic oxide coatings in the marine atmosphere and sea water has been confirmed by many years of operation of oxidized aluminum parts.
• anodic oxidation reduces corrosion of aluminum in acetylene, sulfur dioxide, boric acid and benzenesulfonic acid, ethanol and ethanol solutions.
• in the presence of moisture, the coating hydrates along the pore walls with the formation of boehmite or hydrargylite, which contributes to an increase in the weight of the coating, its compaction and a decrease in the corrosion rate over time.
• in chloride-containing environments, the corrosion process has a clearly expressed local character, flowing through the pores of the coating; it is accompanied by the formation of aluminum hydroxychlorides of variable composition, gradually turning into hydroxide, which also contributes to the gradual clogging of pores and slowing down corrosion.
Figure 12 shows corrosion curves for pure aluminum and aluminum with anodic oxide coatings.
Figure 12 - Corrosion curves for pure and anodized aluminum: SAA - water compacted coating, IC - inorganic dye filled coating, BD - organic dye filled coating, EC - electrochemical painting, Bare Al - pure aluminum. The corrosive medium is 3.5% sodium chloride solution.
For pure aluminum, the corrosion resistance is 0.5953 kOhm, the corrosion current is 130.86 mA. After anodizing, the corrosion resistance increases to 24.216 kOhm, and the corrosion current drops to 7.494 mA.
According to the corrosion curves, it can be seen that the corrosion potential shifts to the negative region in the series SSA, IC, BD, EC, pure Al. In the same series, the corrosion resistance of aluminum decreases.
The atmospheric corrosion rate of pure aluminum is 0.4284 mm/year. After anodizing, the corrosion rate is reduced to 0.0817 mm/year.
Microimages of the surface of anodized aluminum with various types of compaction and filling before and after corrosion are shown in Figure 13.
Figure 13 - Microimages in topographic contrast mode of anodic coatings: SAA - metal anodization with compaction in water; BD - filled with black organic dye; IC - filled with inorganic dye; EC - with electrochemical coloring in tin salts.
Based on practice, the minimum thickness of oxide coatings that provide product protection is selected according to operating conditions:
• in enclosed spaces with artificially controlled climatic conditions - 9 microns;
• outdoors in rural, forest, mountainous areas far from industrial facilities - 15 microns;
• outdoors in urban and seaside atmosphere - 21 microns;
• outdoors in the industrial atmosphere of the northern coast (chlorides not less than 10 mg/m2 * day) with prolonged humidification - 24 microns;
• when applying varnishes and paints, it is allowed to reduce the coating thickness to 9 microns when used outdoors and to 15 microns in a marine atmosphere.
The best corrosion resistance was noted for coatings made on pure aluminum.
Adding copper, silicon, iron, magnesium, and manganese to aluminum improves the mechanical properties of the alloy, but worsens the protective ability of the resulting oxide coatings. Silicon and the intermetallic Al6Mg oxidize much more slowly than aluminum and remain as inclusions in the coating. On the contrary, intermetallic compounds Al3Mg2, Al2Cu, CuAl2, CrAl7, Co2Al9, Co2Al5, Co4Al13, Al7CuFe, Al6CuNi are easily destroyed and increase the porosity of the coating. Thus, the corrosion resistance of coatings with a thickness of 2.5-10 microns obtained on the AD1 alloy is 6-7 times higher than that of coatings on the 1915 and AD31 alloys, and 2-3 times higher than that of coatings on the AMg2AP alloy. Increasing the coating thickness to 15 microns smooths out these differences.
The corrosion resistance of oxide coatings increases with increasing thickness of the barrier layer, which accounts for approximately 1/3 of the corrosion resistance provided. At the same time, increasing the thickness of the porous part of the coating has a beneficial effect on their corrosion resistance only in the case of relatively thin coatings, while a further increase in thickness is accompanied by an increase in pore diameter and a decrease in protective properties.
The porosity of coatings obtained under various conditions is shown in the table below:
| Electrolyte | Working temperature | Bath voltage | Number of pores per 1 m2 n*1012 |
| Sulfuric acid (15%) | 10 | 15 20 30 | 79,1 53,1 28,4 |
| Chromic acid (3%) | 29 | 20 40 60 | 22,2 8,28 4,29 |
The porosity of the surface oxide layer varies from 15 to 40% depending on the grade of the alloy and the anodizing mode. As the electrolyte temperature increases, porosity increases.
The corrosion resistance of films increases slightly with increasing thickness, but the accompanying increase in the coating's porosity and the formation of cracks in the surface layer sharply increases the rate of corrosion damage.
The protective ability of anodic oxide coatings can be significantly improved by filling the pores in various solutions containing substances that inhibit corrosion. A promising way to increase the corrosion resistance of anodic-oxide coatings is the creation of combined coatings in which the porous oxide plays the role of an adsorption layer that holds an organic polymer material that is resistant to aggressive environments.
5.2 Electrophysical properties of aluminum oxide.
Protective and decorative oxide coatings obtained in aqueous solutions of sulfuric acid have high electrophysical characteristics. The microhardness of oxides obtained at a current density of 0.5-2 A/dm2 is N 300 - 500, while that of commercially pure aluminum is about N 30. The microhardness of anodic films, measured on a PMT-3 microhardness tester, on technical aluminum can reach H 600 , and on chemically pure aluminum - H 1500. At the same time, the microhardness of the resulting coatings is uneven in thickness: the layers adjacent to the metal have a microhardness 50-100% higher than the outer ones, which is due to the greater porosity of the surface layers.
The microhardness of functional anodic oxide coatings depends on the nature of the aluminum alloy and is (GPa):
• on pure aluminum - 4.9-5.1,
• on AB alloy - 4.7-4.9,
• on AL type alloys - 4.4-4.7,
• on alloy D16 - 3.24-3.53.
The highest quality coatings are formed on pure aluminum and its alloys with magnesium, the lowest quality - on alloys with a copper content of over 4.5% (D1, D16, D20).
Anodic oxide is a good dielectric: the resistivity is on average 4∙1015 Ohm∙cm, the breakdown voltage can reach 1 kV or more.
To improve the strength and electrical insulating properties, thickened coatings are obtained (usually 40 - 90 microns, although oxidation to a thickness of several tenths of a millimeter is possible). In some industries (instrument making, mechanical engineering, aviation technology), the thickness of coatings is limited to 75 microns due to the possibility of cracks in thick coatings and defective coatings on parts with sharp edges, which sharply reduces the electrical insulating ability and wear resistance.
The electrical properties are affected not only by thickness and porosity, but also by the structure of the coating, so the results strongly depend on the composition of the electrolyte and the processing mode.
Thus, coatings obtained in a sulfate solution with modifying additives on AD0 aluminum 84 μm thick with a porosity of 14% had a breakdown voltage of 2.5 kV, while coatings 165 μm thick with the same porosity were only 1.5 kV.
Films 161 µm thick with a porosity of 9% showed a breakdown voltage of 1.83 kV, and coatings 154 µm thick with a porosity of 23% showed a breakdown voltage of 2.33 kV.
The breakdown voltage on cast alloys is lower than on wrought alloys.
The thermal insulation properties of oxidized aluminum alloys are higher compared to non-oxidized metal. The thermal conductivity of aluminum oxide is 0.004-0.012 J/(cm∙s∙°C), which is 200-500 times lower than that of pure aluminum. The thermal emissivity of an anodized surface is 10 times higher than that of pure metal.
Thick films on aluminum alloys have increased resistance to high temperatures, withstanding heating up to 2000°C. Therefore, oxidation is used in the manufacture of molds for casting aluminum and magnesium alloys. With prolonged repeated exposure to high temperatures, microcracks form on the oxidized surface due to the difference in the values of the linear expansion coefficient of the anodic film and aluminum.
The growth of the oxide layer is associated with partial dissolution of the base metal and the surface layer of the resulting oxide, which affects the change in the size of the part during processing. In the first half hour of treatment, the size of the oxidized part increases by 1 - 2 microns, but then the size begins to decrease to -2 microns after 1 hour of treatment and beyond. Therefore, when applying thicker coatings, it is necessary to take into account the change in the size of parts during the coating process. The increase in the size of the part during anodic oxidation of aluminum and its alloys is less than the thickness of the resulting coating. Typically, the increase in part size ranges from 30 to 60% of the resulting oxide layer thickness in various electrolytes (50% on average).
Aluminum anodizing
Recently, structures and products made from anodized (abbreviated as anod.) aluminum profiles are increasingly in demand. Due to their beautiful appearance and benefits, anodized aluminum products are used in various fields of design and construction.
The oxide (anodic) film cannot protect the metal from the destructive effects of corrosion due to its large porosity, small thickness and low mechanical strength.
The most proven and reliable method of protecting metal and its alloys from harmful corrosion is the process of anodic oxidation in sulfuric acid solutions. This process is also called aluminum anodizing. The oxide layer, which is obtained by electrolytic method, has a density 200-2000 times greater than that of natural oxide films. Compared to other coating methods (varnishing, painting, covering the surface with polymer films), anodizing aluminum in black or any other color eliminates the problems of peeling and under-film corrosion.
Compaction and painting of anodic oxide films on aluminum.
The significant porosity of the oxide coating means that it easily adsorbs moisture, various solutions and organic substances. Porosity during anodic oxidation plays a positive role as a necessary factor for increasing the thickness of the oxide layer, however, during operation, open pores are the weak point of the coating, along which the corrosion process will primarily occur. Therefore, after the formation of a porous oxide, it must be subjected to additional processing designed to close the pores - either with hydrated aluminum oxide (when compacted with water, in inorganic and organic substances), or with various varnishes, oils and other substances with appropriate impregnation.
The ability to adsorb organic substances underlies the coloring process of anodic oxide coatings (Figure 14, 15).
Figure 14 - Examples of anodized aluminum parts filled with black dye.
Transparent and translucent protective and decorative coatings of aluminum and its alloys are painted with aqueous direct acidic organic dyes. The color of films obtained in different anodizing electrolytes varies due to differences in structure, porosity and natural color of the coatings. To obtain the required colors, mixtures of aniline dyes are used. In addition to organic dyes, inorganic dyes are also used. Thus, a limited color range, but greater light resistance, of anodic oxide coatings is obtained by a double exchange reaction in solutions of inorganic salts.
Figure 15 - Examples of anodized aluminum parts filled with turquoise, violet and red dye.
The corrosion resistance of aluminum and its alloys (especially in water and aqueous environments) can be significantly increased by compaction in a solution of chromium salts. Sodium salt is usually used due to economic feasibility. Compositions for compacting anodic oxide coatings in bichromates are regulated by technical specifications DEF151 and are based on work originally carried out in the USSR and the USA.
There are compositions based on sodium bichromate with sodium carbonate or hydroxide and based on sodium bichromate. Treatment in the first solution to seal anodized aluminum lasts 5-10 minutes. This time is not enough to completely compact the anodic oxide film by hydration, but it ensures the absorption of a significant amount of chromate. The anodic coating then turns yellow. The intensity of the yellow color increases depending on the thickness of the coating.
The second composition for compacting anodized aluminum in bichromate without other additives involves processing during the time that was spent on the anodizing itself. This composition provides a satisfactory degree of hydration, but not necessarily complete compaction.
Warm and cold anodic oxidation
Depending on the temperature at which the treatment is carried out, anodic oxidation can be warm or cold. Warm is carried out at room temperature - from +15 to +20 degrees. First, the part is degreased and secured on a special suspension, then immersed in an aqueous electrolyte and kept until a shade similar in color to milk. Then they are washed in cold water, placed in hot aniline dye, and then left for another 30 minutes so that the layer is properly fixed.
Anodic oxidation of aluminum using the “cold” principle is a more labor-intensive, but also more effective procedure. In this case, processing takes place at temperatures from -10 degrees (lower threshold) to +10 (upper threshold).
This method makes it possible to achieve special strength of the anode layer and greater thickness. The result is an almost universal coating.
It has only one drawback: intolerance to organic dyes. But they are not needed, since cold oxidation can give aluminum very beautiful shades: black, grayish and even olive.
After degreasing and anodizing the part in a bath, it is washed with hot or cold water, and then the formed layer is fixed by steaming the aluminum or boiling it in distilled water.
Features of aluminum anodizing
Aluminum anodizing
- the main finishing operation of this lightweight and practical material. Anodic oxidation, as this technology is also called, consists of creating a thin and very strong film of oxides on the metal. This film protects the base metal and plays an important decorative role. It can be made using technologies of different quality and cost.
The anodized coating has a predetermined color. One of the most popular is a color close to the natural tone of aluminum, light silver. Anodizing is performed after any operations involving the deformation of workpieces. Most often, moldings are anodized - pipes, angles, strips, and shaped profiles.
Processing of other metals associated with deformations is carried out in a similar way - all finishing operations are performed after such processing. For example, rolling an I-beam or channel precedes all other work with these rolled profiles. Particularly precise is the work of rolling copper pipes, which is usually performed on short bends - corners and other shaped products. You can learn more about copper pipe bending here.
Colored anodic coatings.
Coatings can be colored not only by filling them with organic and inorganic dyes. They can also be colored directly from certain electrolytes.
If in these electrolytes aluminum and its alloys are anodized first with alternating and then direct current, the coatings are colored from light straw to golden and bronze.
Another method of coloring anodized aluminum is electrochemical treatment in tin or nickel salts.
general information
Chemical oxidation of aluminum is the most accessible, cheap and simple way to obtain oxide films on aluminum and its alloys. The chemical oxidation method does not require an electric current supply. The process is carried out in chromate solutions and allows for the oxidation of a large number of parts at the same time. Oxide films are significantly inferior in protective properties and wear resistance to films obtained by anodic oxidation. The thickness of the oxide layers is about 2 – 3 microns
.
The color of the film obtained in a solution containing sodium fluorosilicate ranges from yellow-golden to brown-golden. The color of the film obtained in a solution containing phosphoric acid on parts made of aluminum and low-alloy alloys is light green, on alloyed alloys it is darker. As the solution is depleted, the color of the film becomes gray-green.