History of the discovery of oxygen
The discovery of oxygen is credited to Joseph Priestley. He had a laboratory equipped with instruments for collecting gases. He tested its physiological effects on himself and on mice. Priestley found that after inhaling the gas, a pleasant lightness was felt for some time. Mice in a hermetically sealed jar of air suffocate faster than in a jar of O2. Since Priestley was an adherent of the phlogiston theory, he never found out what was in his hands. He only described this gas, without even realizing what he was describing. But the laurels of the discovery of oxygen belong to Antoine Laurent de Lavoisier, who gave it its name.
Lavoisier staged his famous experiment, which lasted 12 days. He heated mercury in a retort. When boiling, its red oxide was formed. When the retort was cooled, it turned out that the air in it had decreased by almost 1/6 of its volume, and the remainder of the mercury weighed less than before heating. But when the mercury oxide was decomposed by strong calcination, everything returned: both the lack of mercury and the “disappeared” oxygen.
Subsequently, Lavoisier established that this gas is part of nitric, sulfuric, and phosphoric acids. He mistakenly believed that O2 was necessarily part of acids, and therefore called it “oxygenium,” which means “acid-producing.” Nowadays, acids devoid of “oxygenium” are well known (for example: hydrochloric, hydrogen sulfide, hydrocyanic, etc.).
Being in nature
Accumulation of O2 in the Earth's atmosphere. The green graph is the lower estimate of the oxygen level, the red graph is the upper estimate. 1
.
(3.85-2.45 billion years ago) - O2 was not produced 2
.
(2.45–1.85 Ga) O2 was produced but absorbed by the ocean and seafloor rocks 3
.
(1.85-0.85 billion years ago) O2 leaves the ocean, but is consumed during the oxidation of rocks on land and during the formation of the ozone layer 4
.
(0.85–0.54 billion years ago), all rocks on land were oxidized, and O2 began to accumulate in the atmosphere 5
. (0.54 billion years ago - present) modern period, O2 content in the atmosphere has stabilized
Oxygen is the most common element in the earth's crust; its share (in various compounds, mainly silicates) accounts for about 47% of the mass of the solid earth's crust. Sea and fresh waters contain a huge amount of bound oxygen - 85.82% (by mass). More than 1,500 compounds in the earth's crust contain oxygen.
In the atmosphere, the content of free oxygen is 20.95% by volume and 23.10% by mass (about 1015 tons). However, before the appearance of the first photosynthetic microbes in the Archean 3.5 billion years ago, it was practically absent from the atmosphere. Free oxygen began to appear in large quantities in the Paleoproterozoic (3-2.3 billion years ago) as a result of a global change in the composition of the atmosphere (oxygen catastrophe). For the first billion years, almost all the oxygen was absorbed by iron dissolved in the oceans and formed deposits of jaspilite. 3-2.7 billion years ago it began to be released into the atmosphere and 1.7 billion years ago reached 10% of the current level.
The presence of large amounts of dissolved and free oxygen in the oceans and atmosphere led to the extinction of most anaerobic organisms. However, cellular respiration with oxygen allowed aerobic organisms to produce much more ATP than anaerobic organisms, making them dominant.
Since the beginning of the Cambrian 540 million years ago, oxygen content has fluctuated from 15% to 30% by volume. By the end of the Carboniferous period (about 300 million years ago), its level peaked at 35% by volume, which may have contributed to the large size of insects and amphibians at this time.
The bulk of the oxygen on Earth is produced by the phytoplankton of the World Ocean. About 60% of the oxygen used by living beings is spent on the processes of rotting and decomposition, 80% of the oxygen produced by forests is spent on rotting and decomposition of forest vegetation.
Human activity has very little effect on the amount of free oxygen in the atmosphere. At current rates of photosynthesis, it will take about 2,000 years to restore all the oxygen in the atmosphere.
Oxygen is part of many organic substances and is present in all living cells. In terms of the number of atoms in living cells, it is about 25%, and in terms of mass fraction - about 65%.
In 2021, Danish scientists proved that free oxygen was part of the atmosphere already 3.8 billion years ago.
Methods for producing oxygen
Oxygen is mainly obtained in three ways:
- air separation by low-temperature rectification (deep cooling);
- decomposition of water by electrolysis (passing electric current);
- chemical method.
It is obtained from atmospheric air by deep cooling, as a by-product in the production of nitrogen.
O2 is also produced by passing an electric current through water (water electrolysis) and producing hydrogen along the way.
The chemical method of production is low-productive and, therefore, uneconomical; it has not found wide application and is used in laboratory practice.
Probably many people remember the chemical experiment when potassium permanganate (potassium permanganate KMnO4) is heated in a flask, and then the gas released during the heating process is collected in another flask?
2KMnO4 = K2MnO4 + MnO2 + O2 ↑
And the whole trick was when a smoldering splinter was placed in this flask and it flared up with a bright flame and the teacher explained that the gas released was O2, which supported combustion. And that the combustion process is nothing more than an oxidation process.
Thermal dissociation.
An important laboratory method for producing oxygen, proposed by J. Priestley, is the thermal decomposition of heavy metal oxides: 2HgO ® 2Hg + O2. To do this, Priestley focused the sun's rays on mercury oxide powder. A well-known laboratory method is also the thermal dissociation of oxo salts, for example potassium chlorate in the presence of a catalyst - manganese dioxide:
Manganese dioxide, added in small quantities before calcination, allows maintaining the required temperature and dissociation rate, and the MnO2 itself does not change during the process.
Methods for thermal decomposition of nitrates are also used:
as well as peroxides of some active metals, for example:
2BaO2® 2BaO + O2
The latter method was at one time widely used to extract oxygen from the atmosphere and consisted of heating BaO in air to form BaO2, followed by thermal decomposition of the peroxide.
The thermal decomposition method remains important for the production of hydrogen peroxide. SOME PHYSICAL PROPERTIES OF OXYGEN
| SOME PHYSICAL PROPERTIES OF OXYGEN | |
| Atomic number | 8 |
| Atomic mass | 15,9994 |
| Melting point, °C | –218,4 |
| Boiling point, °C | –183,0 |
| Density | |
| solid, g/cm3 (at temperature ) | 1,27 |
| liquid g/cm3 (at point ) | 1,14 |
| gaseous, g/dm3 (at 0° C) | 1,429 |
| air relative | 1,105 |
| criticala, g/cm3 | 0,430 |
| Critical temperaturea, °C | –118,8 |
| Critical pressurea, atm | 49,7 |
| Solubility, cm3/100 ml of solvent | |
| in water (0° C) | 4,89 |
| in water (100° C) | 1,7 |
| in alcohol (25° C) | 2,78 |
| Radius, Å | 0,74 |
| covalent | 0,66 |
| ionic (O2–) | 1,40 |
| Ionization potential, V | |
| first | 13,614 |
| second | 35,146 |
| Electronegativity (F=4) | 3,5 |
| a Temperature and pressure at which the densities of gas and liquid are the same. | |
Application of oxygen
In addition to the fact that all living beings in nature, with the exception of a few microorganisms, consume oxygen when breathing, it is widely used in many industries: metallurgical, chemical, mechanical engineering, aviation, rocket science and even in medicine.
In the chemical industry it is used:
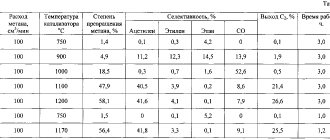
- when producing acetylene from natural gas (methane);
- in the production of acids (nitric, sulfuric);
- for gasification of solid fuel;
- for the production of ammonia, formaldehyde and methanol.
In metallurgy it is used:
- when obtaining non-ferrous metals from ores;
- when smelting cast iron in blast furnaces;
- when smelting steel in open-hearth and electric furnaces;
- oxygen-converter steel smelting.
For medical purposes, patients whose normal functioning of the respiratory or circulatory system is disrupted are artificially increased the O2 content in the air or allowed to breathe pure O2 for a short time. Medical oxygen produced by GOST 5583 is especially carefully purified from all impurities.
Use of oxygen in welding
O2 itself is a non-flammable gas, but due to its ability to actively support combustion and increase the intensity (intensification) of combustion of gases and liquid fuels, it is used in rocket power plants and in all gas-flame processing processes. In gas-flame processing processes such as gas welding and surface hardening, a high flame temperature is achieved by burning flammable gases in O2, and in gas cutting, it causes oxidation and combustion of the metal being cut.
In semi-automatic welding (MIG/MAG), oxygen O2 is used as a component of protective gas mixtures with argon (Ar) or carbon dioxide (CO2).
Oxygen is added to argon during semi-automatic welding of alloy steels to ensure stability of the arc and jet transfer of molten metal into the weld pool. The fact is that, as a surface active element, it reduces the surface tension of the liquid metal, promoting the formation of smaller droplets at the end of the electrode.
When semi-automatically welding low-alloy and low-carbon steels, O2 is added to carbon dioxide to ensure deep penetration and good formation of the weld, as well as to reduce spatter.
Most often, oxygen is used in gaseous form, and in liquid form it is used only during its storage and transportation from the manufacturer to consumers.
Chemical properties
Oxygen gas is an oxidizing agent. By itself, it is non-flammable, but it supports the combustion process well, and at significant concentrations and high temperatures it is explosive.
It can react with active substances (for example, alkali metals) even at room temperature and at ordinary concentrations in the air, forming oxide compounds with them. The result is clearly visible on many metals, on which it manifests itself in the form of corrosion.
Liquid oxygen also has strong oxidizing properties. Many substances impregnated with it are easily flammable and burn, releasing energy and heat. Cotton, paper, wood, coal and some other materials can explode.
Oxygen storage and transportation
Technical and medical gaseous oxygen is produced in accordance with GOST 5583.
It is stored and transported in steel cylinders GOST 949 under a pressure of 15 MPa. Oxygen cylinders are painted blue with the inscription “OXYGEN” in black letters.
Liquid oxygen is produced in accordance with GOST 6331. O2 is in a liquid state only when received, stored and transported. For gas welding or gas cutting, it must be converted back into a gaseous state.
Safety precautions when working with liquid oxygen
When working with liquid oxygen there is no threat of poisoning, but still some safety requirements must be strictly observed:
- wear special clothing to protect parts of the body from frostbite,
- avoid contact with open flames during and 20-30 minutes after working with O2,
- carry out welding and repair work only 2-3 hours after finishing manipulations with this type of gas,
- Before pumping O2, it is necessary to slightly cool the system by using a small amount of product.
Characteristics of oxygen
O2 characteristics are presented in the tables below:
Conversion coefficient for volume and mass of O2 at T=15°C and P=0.1 MPa
| Weight, kg | Volume | |
| Gas, m3 | Liquid, l | |
| 1,337 | 1 | 1,172 |
| 1,141 | 0,853 | 1 |
| 1 | 0,748 | 0,876 |
Conversion factors for volume and mass of O2 at T=0°C and P=0.1 MPa
| Weight, kg | Volume | |
| Gas, m3 | Liquid, l | |
| 1,429 | 1 | 1,252 |
| 1,141 | 0,799 | 1 |
| 1 | 0,700 | 0,876 |
Oxygen in a cylinder
| Name | Cylinder volume, l | Mass of gas in the cylinder, kg | Gas volume (m3) at T=15°C, P=0.1 MPa |
| O2 | 40 | 8,42 | 6,3 |
Thanks to this table, you can now easily answer questions that welders often ask:
- How much oxygen is in the cylinder per m3? Answer: in a 40 liter cylinder there are 6.3 m3
- How much oxygen is in the cylinder? Answer: in a 40 liter cylinder there are 6.3 m3 or 8.42 kg
- How much does an oxygen cylinder weigh? Answer: 58.5 kg - the mass of an empty carbon steel cylinder according to GOST 949; 8.42 - kg mass of oxygen in the cylinder; Total: 58.5 + 8.42 = 69.92 kg weight of the oxygen cylinder.
In order to approximately find out how much oxygen is in the cylinder, you need to multiply the capacity of the cylinder (m3) by the pressure (MPa). For example, if the capacity of the cylinder is 40 liters (0.04 m3), and the gas pressure is 15 MPa, then the volume of oxygen in the cylinder is 0.04 × 15 = 6 m3.
Temperature - boiling - liquid oxygen
The boiling point of liquid oxygen is 182 9 C, argon - 186 1 C. Due to the proximity of these temperatures, it is quite difficult to separate them, however, using multiple rectification, a gas is obtained containing 45 - 50% argon, 45 - 50% oxygen and about 5% nitrogen. To free argon from oxygen, zeolite is also used - a synthetic silicate of aluminum and sodium, which is a molecular sieve. Oxygen molecules pass through the zeolite pores ( d - 2 8 A), and argon molecules are retained. Argon is also obtained from waste from nitrogen fertilizer plants. Ag is used for illuminated advertising as a protective medium. [1]
Hazards and safety precautions
- oxygen is not toxic, in itself is not explosive or flammable, but is a strong oxidizing agent and actively supports the combustion of various materials, especially organic and other flammable substances; therefore, for work in contact with oxygen, only materials approved for this purpose should be used;
- when compressed oxygen under a pressure of more than 30 kgf/cm 2 comes into contact with fats and oils, their instant oxidation occurs, accompanied by the release of heat, which can lead to their ignition, and under certain conditions, to an explosion; in this regard, when working with oxygen, it is necessary to ensure that cylinders, equipment and personnel clothing do not contain traces of fats and oils;
- substances such as wood, coal, paper, asphalt, etc., saturated with liquid oxygen, can detonate;
- to avoid fires, the oxygen content in the air of working premises should not exceed 23% by volume; rooms in which the volume fraction of oxygen may be exceeded must be equipped with exhaust ventilation and air control means; in such premises it is necessary to limit the presence of people and exclude the presence of flammable substances;
- after being in an environment with a high oxygen content, it is forbidden to approach the fire, smoke, it is necessary to ventilate clothes for 30 minutes;
- liquid oxygen affects the mucous membrane of the eyes, and if it comes into contact with the skin, it causes frostbite to the tissue; sampling of liquefied gas must be carried out wearing protective glasses and gloves;
- cylinders and pipelines intended for transporting oxygen cannot be used for storing and transporting other gases; It is necessary to take measures to prevent contamination of cylinders with oil, their collisions, falls, and they also need to be protected from heating by heat sources and precipitation.