History of discovery
This substance has been known to mankind for many hundreds of years. It is known that the Incas and Mayans made balls from rubber for playing ball. Archaeologists found them during excavations, and their age reached 900 years.
Europeans learned about this material much later. Columbus in 1493 in Haiti saw the natives playing with a ball made of rubber.
When the Spaniards took them in their hands, they found that the rubber was sticky and heavy, and smelled of smoke. To make such balls, local residents collected milky juice from the Hevea tree. Balls were rolled out of it and the product was allowed to thicken.
The use of unusual material was not limited to this. The Indians made galoshes from it. Although they did not allow water to pass through, in the heat they began to melt and stick to their feet. If it turned out that these shoes stretched, then they never compressed enough to fit their previous size.
Columbus brought samples of rubber to Europe, but for a long time there it was not possible to produce objects similar to those used by the Indians.
For two centuries, this material remained a curiosity until, in 1730, British chemist Joseph Priestley discovered that rubber could erase what was written with a graphite pencil. In 1791, a businessman from England, Samuel Peale, received a patent for a method he invented for treating clothing, making it waterproof using rubber. Beginning in 1820, France learned to use this material to make garters for women and suspenders for men. For this purpose, rubber threads were used, which were woven with fabric.
British scientist Charles Mackintosh came up with the idea that a layer of rubber could be placed between layers of fabric and thus create a waterproof material for making raincoats. In 1823 he began the production of such clothing. Unfortunately, a cloak made in this way could not withstand cold or heat. In the first case, it became stiff, and in the second, it began to creep apart.
Scientists began to look for ways to make a material from rubber that would be devoid of these disadvantages. American inventor Charles Goodyear solved this problem in 1839 by adding sulfur to rubber.
It turned out that if you put a fabric covered with rubber on the stove, and then apply a layer of sulfur and heat it up, the resulting material will be free from these disadvantages.
The enrichment of rubber with sulfur became known as vulcanization. As a result, rubber was obtained, which began to be actively used. By 1919, there were about 40 thousand different types of rubber products.
The difference between rubber and rubber is as follows:
- rubber has a high level of elasticity, strength, and resistance to adverse effects;
- rubber is valuable primarily not for its performance qualities, but for the fact that it is a raw material for the production of rubber.
Do you know which city produces rubber in Russia? This is Yaroslavl. The plant has been operating since 1932.
An oil field is a source of not only “black gold”, but also numerous by-products - APG, in particular. And instead of burning it in flares, polluting the atmosphere, this gas can be put to use. We saw with our own eyes how this happens in rubber production, looking at the work of the Krasnoyarsk plant.
SIBUR's industrial enterprises, one of which is the Krasnoyarsk Synthetic Rubber Plant (KZSK), process associated petroleum gas (APG) into various products.
We saw how special-purpose rubbers were born. Production, although petrochemical, treats nature very delicately.
The company does not take anything from the land. It processes associated gas that oil workers previously simply destroyed. And he makes a lot of useful things out of it.
Helped us
Alexander Berestyuk, a specialist with 15 years of experience, has worked his way up from a polymerization operator to a leading process engineer in the KZSK technological department
Rubber production in Russia
“We can say that SIBUR enterprises have a responsibility. After all, we are fulfilling an environmental mission by converting associated gas into a wide variety of products. At KZSK it is nitrile butadiene rubber .
Rubber products based on it are resistant to work in aggressive environments and are used in various industries: aircraft manufacturing, tractor manufacturing, mechanical engineering, cable industry. NBR is used in products made from polyvinyl chloride, ABS plastics and other polymer materials.
They are used for the manufacture of sealing elements, packaging materials and much more,” says our guide, leading process engineer of the plant’s technical department, Alexander Berestyuk.
But before reaching the consumer, the rubber production process undergoes a number of transformations, which we were able to see at the enterprise.
The starting point, that is, the raw materials for this production are butadiene and nitrile acrylic acid. It arrives along the railway line - to the loading and unloading trestle in tanks and tank containers. Nitrile-acrylic acid is placed in special storage containers, which are placed in a row near the overpass. A few meters away from them are containers with butadiene.
Butadiene in Krasnoyarsk is “related”: it is supplied from the Tobolsk production plant of SIBUR.
“We are part of an integrated company: SIBUR carries out a processing cycle - from associated petroleum gas to downstream products.
It turns out to be a chain: APG is purchased from oil producers, and NGLs are extracted from it at gas processing plants, which is sent for processing to Tobolsk.
Various products are obtained from NGLs, including butadiene, which is supplied to the production of synthetic rubbers - to us or to factories in Voronezh and Tolyatti. Everyone has their own specialization.
By the way, the first production of nitrile butadiene rubber was opened in Krasnoyarsk. Over the years of operation, the enterprise has mastered the production of over 85 brands of rubber,” Alexander Berestyuk describes part of the mentioned chain.
At this time, movement began on the loading and unloading trestle - a new batch of butadiene arrived. It must be said that the plant was initially designed wisely and with great prospects. The location was chosen very well: there is a railway nearby, as well as a thermal power plant that supplies the necessary energy resources.
We asked how energy-intensive rubber production is - it’s not for nothing that such a neighborhood has been formed. It turned out to be enough.
However, the world does not stand still, technology is improving, and at each stage our guide talked about new and modernized installations that reduce energy and steam consumption.
Improving production also helps increase volumes: last year the plant produced 43,300 tons of rubber, and plans for this year are already 46,000 tons. This is precisely the permitted capacity of the plant today, which is gradually increasing over time.
Invisible transformations
Meanwhile, we moved to the next production site - to the polymerization unit, where the hydrocarbon mixture is mixed with the aqueous phase and sent to reactors for the polymerization process.
They explained to us that the aqueous phase, relatively speaking, is a solution of soap, which additionally contains the components necessary for synthesis.
When mixed, an emulsion is obtained, which is fed into the polymerization battery. The initiator solution is also supplied here. As a result, the emulsion gradually turns into latex - tiny particles of nitrile butadiene rubber, which are distributed in an aqueous environment and stabilized by an emulsifier.
However, we did not see all these miracles: the polymerization department looks like a room filled from floor to ceiling with intertwining pipes - of different diameters and with assorted shut-off valves. Unless the signs with the names of the components give away chemical production.
The latex, after removing unreacted monomers at the degassing stage, is fed into storage tanks, where it is averaged, and a batch with the required quality indicators is formed from it.
In the laboratory, specialists carry out the necessary tests, and if everything is in order, the batch of latex is sent to the next stage - the isolation stage.
Getting closer to rubber
People call the apparatus into which the latex obtained at the previous stage arrives a “guitar”; professionals call it a system of variable-section jet apparatuses.
A coagulant is also supplied here - a substance that releases rubber from latex.
A little later, already in the laboratory, Alexander Berestyuk showed the process in miniature: he added a coagulant solution to latex, and from two liquids - transparent and milky white - a piece of rubber was obtained. In production, everything looks about the same, only on a scale and with the participation of specialized equipment.
Extraction of rubber from latex. production process in miniature
After the coagulation process, the wet rubber is broken into crumbs, which are fed to a vibrating sieve.
Here the primary separation of moisture occurs, then the rubber crumbs are washed and fed to the next vibrating sieve. Here the product is almost ready - you can even touch it.
We held it in the palm of our hand and were surprised at how different the white lumps (soft pinkish shavings) were from finished rubber goods products.
Although the rubber has passed through the vibrating screens, it is still wet. Therefore, we need an expeller - a squeezing machine.
“The design resembles a large meat grinder. Cylindrical body, screw, plate with holes. Only in a meat grinder the knife is on the inside, while in ours it is on the outside to cut off the crumbs of rubber. Then it falls down, and there is another expeller,” shows Alexander Berestyuk.
On the vibrating screen, the moisture content of the material is about 70-80%. After the first expeller it’s already 10%, after the second it’s no more than 5%.
Our guide explained: if the rubber is not squeezed out, you will have to dry it for 7-10 hours, and the dryer will be several kilometers long. Otherwise it only takes 30 minutes and a few meters of drying.
Yes, the dryer is the next stop on the rubber's journey. Using a vibration distributor, it is evenly distributed along the dryer conveyor, and a solid carpet of rubber crumbs is sent to a chamber in the form of a corridor, where it is dried with hot air. The output is soft pinkish chips - no more than 0.5% moisture. Next is a vibratory lifter and a press, where a finished briquette weighing 30+/- 0.5 kg is formed.
Pipelines for transporting raw materials are made of ordinary, so-called “black” steel. But in the polymerization shop, stainless steel is already used: latex has high adhesion, forms polymer deposits that have to be cleaned - this is easier to do with stainless steel pipes.
“After pressing, the briquette passes through a metal detector to ensure that there are no foreign metal inclusions in the material; then, through a conveyor scale, the rubber goes to the stage of packaging in plastic film and is marked,” Alexander Berestyuk describes the finishing touches.
And now, finally, we see what this whole clever structure was organized for: briquettes of finished rubber are placed in containers.
“Touch it! — the workers say, laughing. “Fresh, still warm!”
The containers are sent to a warehouse, and from there the rubber is distributed to rubber goods factories in 35 countries around the world.
Golden hands and heads
You might think that a whole city works in production with so many stages. There are really a lot of employees at the plant - 390 people. However, the workshops are virtually deserted - in some places completely empty.
Production is automated as much as possible, so that workers only control the process of its work - for the most part, remotely.
That is why there are so many reflexive verbs in our story: no one washes the rubber and adds ingredients: everything is washed and mixed - as if on its own.
While we were in the polymerization department, Alexander Berestyuk drew our attention to the possibilities of automatic control.
“Here, in the workshop, there is a flow meter, the information is sent to the process control system and compared with the parameters set by the polymerization operator or the shift supervisor.
If the value is greater or less, the process control system changes it using a control valve.”
Last and first
The government decree on the construction of a synthetic rubber plant on the territory of Krasnoyarsk was signed in 1947.
Construction began two years later, and in 1952 they produced the first roll - then the products were still produced in rolls - of alpha-methylstyrene rubber.
It was a special purpose material - it was used for the needs of the defense industry. At that time, the plant was part of the so-called “golden ring of the chemical industry” - an industrial complex whose enterprises operated in a chain.
But when the unified plant management system disappeared, the “golden ring” also collapsed.
The synthetic rubber plant was the last of a large family left. In the most difficult years, production volumes fell to the very bottom, but the enterprise held on.
In 2001, the plant was acquired by the petrochemical company SIBUR - first 95%, and later 100%. So today KZSK has become part of another team - the first player in this market.
For this purpose, the plant has several control panels. In response to our naive question about how we can see something in these intersecting graphs, tables and diagrams, the representatives of the duty shift only smiled.
They eagerly began to tell us: you see, the work of the entire polymerization installation is displayed here. All values are within specified limits.
If something goes wrong, the system signals: first a warning alarm will go off (that is, nothing critical, but measures should already be taken), and in the case of force majeure - a stop, the valves close, the equipment stops working.
Production parameters are controlled directly from the control panel
“We have a special document - the Emergency Response Plan, which specifies the actions of personnel in any production situations.
Partial regulation occurs directly from here - from the switchboard, but if shutdown is necessary, it is better to close the valve manually - it’s safer. But in any case, this is a very operational process,” the experts explained.
And one more step
At the stage of creating briquettes, the main work of the rubber plant is completed. And for nitrile butadiene rubber itself, everything is just beginning. The fact is that this material is characterized by increased oil and gasoline resistance.
Accordingly, products made from it are in demand where rubber parts (RTI) will have to come into contact with petroleum products - fuel, oils. That is, the key consumers are the auto industry and the oil industry.
The enterprise itself does not produce rubber goods, but the laboratory has set up “miniature production”: test samples of the final product are created so that it is possible to evaluate its properties from the point of view of consumers.
The market will catch up!
Specialists from the synthetic rubber plant confirmed the traditional opinion of analysts that the petrochemical consumption market in Russia is limited, but only in comparison with per capita consumption in more developed economies.
At the same time, in a certain sense, supply can also stimulate demand.
When a large volume of domestic product becomes available, processors have more incentive to expand their production.
In addition, there is also a niche for import substitution. And the products themselves are being improved, and today, for example, polymers are already used where metal and other materials used to work.
The same applies to synthetic rubbers. This market in the world is growing by 3% annually, the demand for butadiene-nitrile rubber is much higher.
Even the demographic situation and income level of the population indirectly influence it.
Let's say, a family needed not one car, but two. And the automobile industry is one of the main consumers of Krasnoyarsk synthetic rubber.
In addition, modern technologies make it possible to create new products with new quality characteristics. Over the past few years, KZSK has mastered the production of many modern brands of products, which has opened up growth prospects for the plant in the markets of Europe, Asia and, of course, Russia.
The plant’s laboratory specialists carry out incoming inspection of raw materials, analyze the quality of intermediate and finished products
Synthetic rubber, as already mentioned, is a light and fairly soft material, it can easily be torn by hand, while rubber goods, as everyone knows, are black, hard, and elastic.
“In the laboratory we prepare a standard rubber compound. Carbon black is added to the recipe for strength, which also gives the final product a black color.
Sulfur is also added - a vulcanizing agent, and other components necessary for the vulcanization process.
The rubber mixture is prepared on laboratory rollers.
They work like this: two rolls rotate in different directions, and they have different rotation speeds. Due to this, all the necessary components are easily introduced into the rubber.
Next, the so-called raw rubber is placed in a mold, and the mold is placed in a vulcanization press, where the vulcanization process occurs by compressing press plates heated to 150 degrees at a certain time. The result is a vulcanizate,” our guide demonstrates the process.
From the resulting material, a sample of the established shape is cut out - similar to a two-bladed oar. It needs to be tested for strength and elasticity: to do this, the sample is inserted right in front of us into a special device - a strain gauge and begins to stretch at a certain speed.
Who would have thought that seemingly hard rubber could stretch so noticeably and then return to its original shape.
They explained to us: according to the technical specifications for the finished products being tested, the sample must withstand a load of at least 250 kgf/cm2 and stretch by 450%. With the force of the machine, the sample is finally broken. The mission is accomplished: the products comply with the standards.
Anna Kuchumova visited the production site Photo: Evgeniy Oshkin
Physico-chemical properties of rubber
This material is an elastic mass that was originally obtained from the Hevea plant. Over time, the milky sap coagulates and forms a viscous material. To prevent this from happening, sodium hydrosulfide or formaldehyde is added to it.
Freshly extracted rubber juice (latex) is characterized by the following properties:
- The specific gravity is 0.9794 (with a rubber content of 35 g per 100 cubic cm).
- At a temperature of 30 degrees Celsius, the viscosity ranges from 12 to 15.
- The size of rubber particles is 0.5-5 microns. In 1 cubic cm of juice, their number reaches 200 million.
Rubber is a polymer of unsaturated hydrocarbons. Its chemical formula is as follows: (C5H8)n - it is an isoprene polymer. The molecular weight of this substance is 15000-30000. After conducting research, scientists found that rubber consists of a polymer of 2-methylbutadiene.
Properties of natural rubber
Macromolecules of natural rubber have a linear structure and are rolled into a ball. When force is applied to rubber, it can stretch, and after the load is removed, it can compress and return to its previous shape. This property of rubber is called elasticity. Due to its elasticity, natural rubber is highly resistant to wear. But as the temperature rises, it becomes sticky, soft and loses elasticity, and also cannot return to its previous state. If the rubber is subjected to further heating, it melts.
However, in the cold, rubber becomes hard and brittle.
Studies have shown that when natural rubber is heated, the main decomposition product is isoprene, a diene hydrocarbon.
Thus, natural rubber is a natural polymer, the macromolecules of which consist of units (-C5H8-).
An example of a reaction scheme for the polymerization of isoprene in the formation of natural rubber:
The presence of a double bond indicates that natural rubber is an unsaturated hydrocarbon.
Natural rubber
99% of this material is obtained from the Hevea tree. To do this, cuts are made on the bark in the shape of the letter V. A groove is installed in the lower part, perpendicular to the surface, through which the juice gradually flows into a bowl placed below. The leakage of latex (milky sap of Hevea) lasts for one and a half hours.
The rubber content in it may vary. It depends on the:
- the age of the tree from which the sap is collected;
- the composition of the soil in which Hevea grows is important;
- the time of year when the collection takes place;
- what the weather was like at that time;
- time and quality of cuts made;
- other features of latex collection.
In order for natural rubber to be used, it must undergo the following processing:
- First the spin is done. It is necessary in order to remove excess moisture from latex.
- After this, the resulting strips are wrapped around a stick and dried over a fire.
- The strips are laid out in one layer and left in the sun.
- Now all that remains is to hold it over the smoke.
Rubber prepared in this way can serve as a raw material for rubber production.
The juice is extracted from trees that are already 12 years old. From 3 to 5.5 kg of latex can be obtained per year.
Composition of latex solution:
- up to 70% water;
- the rubber content in different cases ranges from 25% to 70%;
- the content of other chemicals, including protein, does not exceed 1-2%.
Types and types of natural rubber:
Natural rubber is divided into 8 types, forming 35 varieties.
The most common and valuable type of natural rubber is “ smoked sheet ,” which means smoked leaf. It is made in the form of fairly transparent amber-colored sheets with a corrugated surface.
Less common is the type called " light crepe ". To obtain it, sodium bisulfite is added to the latex before gelatinization for bleaching. Sheets of this type of rubber are cream-colored and opaque.
The least valued type is called " para-rubber ". It is extracted from wild Hevea using an artisanal method.
Synthetic rubber and its main types
Butadiene rubber is used to make automobile tubes and tires. The operational, as well as physical and chemical properties of the products are much better compared to natural materials.
One of its features is the ability to reliably hold air. It exceeds the similar quality of natural material by about 10 times. Chemistry has made it possible to create materials whose characteristics are significantly superior to natural rubber.
Another area of application is the production of ebonite or chemically resistant rubber.
Chloroprene rubber is supplied to customers in the form of a light yellow mass.
Distinctive qualities of the product:
- high resistance to fire and temperature;
- it is resistant to ozone, low temperatures and other types of weather influences;
- it has a high level of adhesion to fabrics, metals and other materials.
The material can crystallize under tension. This quality increases its strength characteristics.
Material made on the basis of ethylene propylene is used where impact-resistant rubber is needed.
Organosilicon rubbers have increased resistance to temperature, chemical influences, and abrasion. This material does not allow gases to pass through.
Divinyl rubber is used to create gaskets in high pressure installations.
Receipt
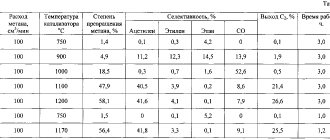
Butadiene rubber is produced by the polymerization reaction of butadiene in a solution of an organic compound, such as toluene, olefins, etc. Butadiene is polymerized using continuous technology in a chain of successive interconnected reactors over several hours. Approximately 90% of the monomer is ultimately converted into polymer.
Fig.1. Synthesis circuit
After the synthesis reaction is terminated, the catalyst is deactivated, antioxidants are added to the system, and the resulting polymer solution is washed. After this, the finished rubber is isolated from the reaction mixture, separated from water, dried, cut into briquettes and packaged.
Obtaining synthetic rubber
When rubber began to be widely used in industry, there was an acute shortage of natural rubber for its production. This situation has confronted scientists with the task of synthesizing an artificial material with the same physical and chemical properties.
Preparation of synthetic rubber using the Lebedev method
The installation for producing this material was first put into operation in the thirties of the 20th century.
Synthetic rubber is made from divinyl, which is extracted using the decomposition reaction of alcohol. The monomer of artificial rubber is isoprene. The material is obtained by polymerization.
What have we learned?
From the 10th grade chemistry lesson we learned about the structure, properties and use of rubbers. Rubber is a natural or synthetic material that is elastic. Natural rubbers are obtained from latex, the viscous sap of some tropical trees. It is produced industrially from alkadienes, in particular from isoprene. Synthetic rubber was first produced in 1932. Physical properties change depending on temperature. The lower the temperature, the more fragile the material. Rubber is made from rubber.
Previous
ChemistryCycloalkanes - general formula, structure
Next
ChemistryPlastic - formula in chemistry
Rubber Applications
In its pure form, this material is rarely used. In most cases, it is used as a base for making rubber.
After rubber was brought to Europe, until the 18th century, rubber was considered simply one of the overseas curiosities. Elasticity and water-repellent properties made it possible to use the material for the manufacture of shoes and clothing that do not allow water to pass through, however, low performance qualities prevented its spread.
After the discovery of vulcanization of rubber, which made it possible to make rubber, the use of the new material became very common. Gradually, the quality of rubber improved and a large number of different products began to be made from it.
Examples include:
- tires;
- children's rubber toys;
- shoes;
- clothes;
- electrical insulation for wires;
- conveyor belts;
- medical products;
- rubber protective gloves.
Now it is difficult to name an area of human life where rubber is not used.
Natural rubber continues to be used today. Tires, shock absorbers, and some products for sanitary and hygienic purposes are made from it.
Application
The main use of rubber is the production of rubber for tires. The material is also used to make:
- heat, electrical, sound, waterproofing materials;
- solid rocket fuel;
- seals;
- glue;
- varnishes;
- elastic bands;
- floor coverings;
- hoses;
- gloves;
- shoes;
- toys;
- furniture;
- erasers.
Rice. 3. Rubber products.
Interesting facts about rubber
After the vulcanization process was discovered, the material began to be actively used in industry. At the same time, the Hevea juice, which was mined in the Brazilian jungle, became scarce.
In order to increase rubber production, large Hevea plantations were established on the islands of Java and Sumatra.
Although the main source of natural rubber is Hevea, nevertheless, in nature there are other options for obtaining this raw material from plants.
Starting material
It is a colorless 1.3-butadiene gas, the formula of which is as follows: CH2=CH—CH=CH2. It is also called divinyl. In fact, butadiene-1.3 is an unsaturated hydrocarbon, a representative of the diene group. A characteristic feature of this gas, among other things, is a very unpleasant odor.
The polymerization of butadiene to produce rubber itself is carried out on stereoscopic catalysts. The reaction itself occurs with the addition of molecules to each other in the 1,4 or 1,2 position.
Properties of rubber and rubbers obtained from it
Chloroprene rubber
Butadiene rubbers are soluble in aromatic and aliphatic hydrocarbons, their chlorinated derivatives, and cyclohexane. The density of BC is usually from 900 to 920 kg/cubic meter. m.
The chemical properties of butadiene rubbers are largely due to the presence of double bonds in the chain. They react with halogens: bromine, chlorine, in addition, with substances that have enough free halogen atoms.
Also, butadiene rubbers can be subjected to hydrogenation with hydrogen dissolved in hydrocarbons, provided that complex catalysts are present in the environment. Rubbers can add thiols and react through the mechanisms of epoxidation, cyclization, etc.
Butadiene rubbers, like many others, are vulcanized mainly with the help of elemental sulfur; in addition, it is possible to use tetramethylthiuram disulfide, organic peroxides and some thermosetting resins. The resulting rubber is filled with carbon black, highly dispersed silicon oxide, chalk or kaolin. Rubbers are plasticized with mineral oils in composition with hydrocarbons of various types.
The main advantage of vulcanizates or rubbers made from butadiene and some other types of rubbers is precisely stereoregular rubbers - they have excellent elastic and wear-resistant properties.
The best complexes of beneficial properties are obtained by using compositions of butadiene and other rubbers with subsequent vulcanization of the mixtures. This way you can achieve increased strength characteristics, tear resistance, excellent elastic characteristics and wear resistance.
Vulcanizates of such rubbers have good gas permeability. The frost resistance of such vulcanizates depends on their ability to crystallize when the temperature drops. There are special ways to improve frost resistance.
Rubber. Chemistry presentation.
Here you can download presentation slides on the topic “Rubber” in chemistry.
Just below there will be information from the images that is used in Wikipedia and other sources (all links are located after the article)
- Rubber is
- Natural and synthetic rubber.
- Vulcanization of rubber.
- Types of rubber.
- Natural rubber.
- Examples of some synthetic rubbers.
- Sources:
Rubber is
Rubbers are natural or synthetic elastomers characterized by elasticity, water resistance and electrical insulating properties, from which rubber and hard rubber are obtained by vulcanization.
At the end of the 15th century, the Indians of North America learned to obtain rubber from the sap of the Hevea tree, which they used in the manufacture of shoes and other things. When the hevea bark was cut, drops of milky white juice—latex—were released. The Indians called this sap “tears of the tree,” which sounds like kau-uchu . Hence the name - rubber.
Natural and synthetic rubber.
The discovery of America by Christopher Columbus contributed to the spread of the wonderful material to Europe, where rubber was first obtained through trial and error. With the advent of the automobile industry in the 20th century, the demand for rubber, and therefore rubber, began to grow. At that time, the cost of rubber products was very high. This is due to the fact that only 1-2 kg of rubber can be obtained per year from one Hevea tree, while the production of, for example, tires required 50 more.
Soon there was a shortage of rubber ( natural rubber ) obtained from the juice of the Hevea plant.
In the 20s of the 20th century, the Russian scientist S.V. Lebedev obtained the first synthetic rubber by polymerizing 1,3-butadiene (divinyl) on a sodium catalyst. Later, the sodium catalyst was replaced by a Ziegler-Natta catalyst (Al(C2H5)3∙TiCl4), which made it possible to obtain polybutadiene and polyisoprene, a synthetic rubber with the necessary properties of elasticity and strength. Synthetic rubber became so popular that by the end of the 20th century it almost completely replaced natural rubber.
Rubber products gained enormous, albeit short-lived, popularity in Europe and North America after the Englishman Chaffee invented rubberized fabric. He dissolved raw rubber in turpentine, added carbon black and, using a specially designed machine, applied a thin layer of the mixture to the fabric. Not only clothes, shoes and hats were made from such material, but also the roofs of houses and vans. However, products made from rubberized fabric had a big drawback. – the elasticity of rubber manifests itself only in a small temperature range, therefore, in cold weather, rubber products hardened and could crack, and in the summer they softened, turning into a sticky, stinking mass.
Preparation - styrene butadiene rubber
The production of styrene-butadiene rubbers using lithium metal differs only in the initial initiation stage, which is carried out in a special apparatus. A mixture of monomers, solvent and molecular weight regulator is continuously fed into the apparatus where large lithium granules are placed. The granules are suspended due to mixing. With vigorous stirring in the presence of monomer and regulator, initiation occurs. The solution containing the active centers of the living polymer enters the battery of polymerizers and then the process is similar to the process using lithium alkyls. The consumption of lithium metal by this method is close to theoretical.
To obtain styrene-butadiene rubber, a standard recipe is used. In the process of improving technology, the ratios of ingredients are periodically revised, although the range of permissible fluctuations of the main parameters remains almost unchanged, which allows us to speak about the commonality of algorithms and control systems for the processes of producing emulsion rubbers and latexes of various brands.
In the practice of producing styrene-butadiene rubber, the most common method is the separation of rubber from latex under the influence of electrolytes. When introducing electrolytes, undesirable impurities may form and remain in the rubber, affecting the properties of the polymer and its vulcanizate and the rate of vulcanization
Therefore, much attention is paid to washing rubber. In the practice of isolating emulsion rubbers, a solution of sodium chloride in combination with various acids—acetic, sulfuric—is most often used as a coagulant; in some cases, aluminum salts are used in combination with sulfuric acid
When coagulating latexes stabilized by alkyl (aryl) sulfonates, magnesium and calcium salts are used; in some cases, a mixture of electrolytes is used as a coagulant, for example NaCl - f A12 (SO4) 3; NaCl MgS04 (MgCl2, CaC12), which is advisable to reduce the consumption of the main electrolyte.
The ratio of monomers and the composition of the charge for producing styrene-butadiene rubbers are determined by the brand of rubber produced.
It is used in the synthetic rubber industry for the production of styrene-butadiene rubbers and latexes, in the plastics industry for the production of polystyrene and styrene copolymers, as well as in paint and varnish, chemical-pharmaceutical and other industries.
One of the rapidly developing areas of polymer synthesis is the production of styrene-butadiene rubbers in solution in the presence of lithium catalysts. One of the reasons for such rapid development is the relative ease of obtaining these rubbers and their value as materials for the manufacture of a wide range of rubber products.
The process of distilling off unpolymerized monomers is similar to the corresponding process in the production of styrene-butadiene rubbers. However, in the production of latexes there are strict requirements for the content of free monomers. When distilling monomers from latex, a direct-flow (for example, for SKS-65GP, SKS-85GP) or counter-current scheme of latex contact with water vapor is used.
Mercaptans are included in the composition to regulate polymerization during the production of styrene-butadiene rubber.
The method for producing nitrile butadiene rubbers (SKN) is similar to the method for producing styrene butadiene rubbers.
| Scheme of emulsion copolymerization of butadiene and styrene. |
In Fig. 244 shows a diagram of emulsion polymerization in relation to the process of producing styrene-butadiene rubber.
| Scheme of emulsion copolymerization of butadiene and styrene. |
In Fig. 244 shows a diagram of emulsion polymerization in relation to the process of producing styrene-butadiene rubber.
Styrene is the main component in the production of polystyrene and a copolymer in the production of styrene-butadiene rubber. They copolymerize with butadiene (75 - 85% butadiene and 15 - 25% vinyltoluenes) to form plastic materials used in the production of heat-resistant and hydrophobic varnishes.
Polymerization is carried out in an aqueous emulsion under conditions close to the conditions for producing styrene-butadiene rubber.