General information
Refrigeration cycle, step 1. The hot refrigerant compressed by the compressor is cooled by the surrounding air and condenses in the heat exchanger of the window air conditioner
Specific volume is volume per unit mass. This property of substances is often used in thermodynamics. Specific volume is the reciprocal of density. It is found by dividing volume by mass. The specific volume of gases can also be found by their density, temperature and molecular weight. The value of volume per unit mass is used more often, but sometimes, speaking of specific volume, they mean the ratio of volume to molecular weight. It is usually clear from the context what specific volume we are talking about. Specific volume units by mass are different from specific volume units by molecular weight, so you can understand what specific volume you are talking about by looking at the units in which it is measured. Specific volume by mass is measured in m³/kg, l/kg, or ft³/lb, while specific volume by molecular weight is measured in m³/mol and derivative units. In some cases, the specific volume by molecular weight is called molar volume
or
specific molar volume
.
Volume, mass, density, specific volume. Reduction to normal and standard conditions and recalculation
Reduction to normal and standard conditions
The unit of gas volume is the cubic meter (m³).
The measured volume is reduced to normal physical conditions. Normal physical conditions: pressure 101,325 Pa, temperature 273.16 K (0 ° C).
Standard conditions: pressure 101,325 Pa, temperature 293.16 K (+20 ° C).
Currently, these designations are falling out of use. Therefore, in the future, it is necessary to indicate those conditions that include volumes and other gas parameters. If these conditions are not indicated, this means that the gas parameters are given at 0 ° C (273.16 ° K) and 760 mm Hg. Art. (1.033 kgf/cm²). Sometimes the volume of gas (especially in foreign literature and standards) when using the SI system is reduced to 288.16 °K (+15 °C) and a pressure of 1 bar (105 Pa).
If the volume of gas under certain conditions is known, then it can be converted into volumes under other conditions using the coefficients given in the following table.
Coefficients for converting gas volumes from one condition to another
| Gas temperature and pressure | 0 °C and 760 mm Hg. Art. | 15 °C and 760 mm Hg. Art. | 20 °C and 760 mm Hg. Art. | 15 °C (288.16 °K) and 1 bar |
| 0 °C and 760 mm Hg. Art. (normal conditions) | 1 | 1,055 | 1,073 | 1,069 |
| 15 °C and 760 mm Hg. Art. (in the early literature) | 0,948 | 1 | 1,019 | 1,013 |
| 20 °C and 760 mm Hg. Art. (Art. conditions) | 0,932 | 0,983 | 1 | 0,966 |
| 15 °C (288.16 °K) and 1 bar (SI) | 0,936 | 0,987 | 1,003 | 1 |
To bring gas volumes to 0 °C (273.16 °K) and 760 mm Hg. Art. (1.033 kgf/cm²), as well as to 20 °C (293.16 °K) and 760 mm Hg. Art. (1.033 kgf/cm²) the following formulas can be applied:
where V0 °С and 760 mm Hg. Art. — volume of gas at 0 °C and 760 mm Hg. st., m³; V20° C and 760 mm Hg. Art. — volume of gas at 20 °C and 760 mm Hg. st., m³; VP—gas volume under operating conditions, m³; р—absolute gas pressure under operating conditions, mm Hg. Art.; T is the absolute gas temperature under operating conditions, °K.
Recalculation of gas volumes reduced to 0 °C and 760 mm Hg. Art., as well as to 20 ° C and 760 mm Hg. Art., in volumes under other (working) conditions can be produced according to the formulas:
Any gas can expand. Consequently, knowledge of the volume occupied by a gas is not sufficient to determine its mass, since in any volume entirely filled with gas, its mass may be different.
Mass is a measure of the substance of a body (liquid, gas) at rest; a scalar quantity characterizing the inertial and gravitational properties of a body. The SI units of mass are kilogram (kg).
Density, or mass per unit volume, denoted by the letter p, is the ratio of body mass m, kg, to its volume, V, m³:
p = m/V
or taking into account the chemical formula of the gas:
p = M/VM = M/22.4,
where M is molecular weight, VM is molar volume.
The SI unit of density is kilogram per cubic meter (kg/m³).
Knowing the composition of the gas mixture and the density of its components, we determine the average density of the mixture using the mixing rule:
pcm = (p1V1 + p2V2 + … + pnVn)/100,
where p1, p2, …, pn is the density of gas fuel components, kg/m³; V1, V2, …, Vn—component content, vol. %.
The inverse of density is called specific, or mass, volume (ν) and is measured in cubic meters per kilogram (m³/kg).
As a rule, in practice, to show how much 1 m³ of gas is lighter or heavier than 1 m³ of air, the concept of relative density d is used, which is the ratio of gas density to air density:
d = p/1.293
And
d = M/(22.4×1.293).
Use of specific volume
If you compare solids, liquids and gases, it is easy to see that changing the density or specific volume of gases is the easiest. By the way, when talking about solids and liquids, they most often use density, and when talking about gases, they often use specific volume. Specific volume is also commonly used when dealing with systems in which a substance or substances are present in several different states of matter.
Refrigeration Cycle Step 2: The cooled refrigerant in liquid form passes through the capillary tube and enters the evaporator (heat exchanger shown in the illustration). Warm air from the room passes through the cold evaporator, where it is cooled
Two-phase systems
Two-phase systems are systems that consist of a substance in two different states of aggregation, for example, liquid-gas or liquid-solid. A mixture of ice and water in a cup is a good example of a liquid-solid system. Liquid-gas systems can be found in a gas-fired power plant boiler, a nuclear reactor, or an air conditioner. In some cases, it is interesting to observe a two-phase system, for example to see how it changes with temperature or pressure. Often of interest are changes in the volume of a substance when the state of aggregation of this substance changes. In this case, specific volume is used. In general, specific volume is convenient to use to describe the properties of a two-phase system.
First, let's look at examples of two-phase systems and their applications in everyday life and technology. We will then discuss the application of specific volume.
Heating, ventilation and air conditioning systems
Refrigeration cycle, step 3. The refrigerant gas leaves the evaporator and enters the compressor, where it is compressed. At the same time, the pressure in the refrigerant increases. Afterwards it enters the condenser (heat exchanger), and the cooling cycle repeats
Most heating, ventilation and air conditioning (HVAC) installations use two-phase systems. In heating, water is sometimes heated until it turns into steam, which is pumped through the heating system pipes to heat the room, condensed in the heating radiators, and returned to the boiler as a liquid. Many heating systems circulate hot water through pipes. In such heating systems, boilers are used to heat water. The water in the boiler is heated by burning fuel. This is often a fossil fuel such as coal or natural gas.
On the other hand, the cooling process uses a substance called a refrigerant or refrigerant. During operation, this substance is alternately in two phases - liquid and gaseous. First, the refrigerant gas is cooled in a heat exchanger called a condenser until it becomes a liquid. The condenser is located outside the refrigerated space. In this case, the refrigerant condenses on the walls of the heat exchanger, releasing heat to the environment. The refrigerant is then compressed by a compressor and passed through pipes through another heat exchanger called an evaporator located in the refrigerated room. It turns liquid refrigerant into gas. This transformation requires a lot of heat, which is removed in the refrigerated room. In gaseous state, the refrigerant is returned to the first heat exchanger and the entire process is repeated.
Outdoor split air conditioning unit
The transition of a liquid into a gaseous state requires a large amount of energy. During the cooling process, the system takes heat from the room to heat the refrigerant, and thereby cools the room. The condenser in the air conditioner cools the gas (refrigerant), releasing heat to the environment, that is, to the street.
Home refrigerators and industrial refrigerators work on the same principle. Some HVAC units are combined into one system. In other cases, the heater and air conditioner are separate units.
Solar collectors are used for cooling
Solar collectors
Solar collectors work on a similar principle. Solar panels collect energy from the sun, which is used to heat air or a liquid such as water or antifreeze. The resulting thermal energy is used to heat rooms or heat water.
Heat pipes are highly efficient heat transfer devices. Their high heat transfer is ensured due to the large amount of energy that is spent on vaporization and released when the liquid condenses inside them
Heat pipes
The process of heat pipes is similar to that of an air conditioner, with the difference that instead of cooling the air, they cool hard surfaces, such as metal. The heat from these surfaces heats the liquid in the tubes until the liquid evaporates. Otherwise the process is identical: the gas cools and condenses and is returned to the tubes to be heated. Examples of coolants are helium, alcohol, and mercury. Often such systems are used inside electronic devices, such as computers, to cool electronic components that are subject to extreme heat. These systems are also used in space under extreme temperature conditions.
Design and operation of two-phase systems
Under certain conditions, a substance in two-phase systems can usually be in that system simultaneously in two different phases. If these conditions are not met, then the substance in the system can only be in one state of aggregation, as we will describe in detail below.
In two-phase systems, temperature changes are caused by changes in pressure, not specific volume. Sometimes, on the contrary, the pressure and temperature are constant, but the specific volume changes. This occurs when a constant pressure in the system maintains a temperature that allows the substance to exist simultaneously in two phases. Under such conditions, as soon as the system reaches the desired temperature, if this temperature does not change, the liquid gradually turns into a gaseous state, and the specific volume increases as a result. Of course, this also changes the total volume of matter in the system. The system itself must also be designed for such an increase in volume. On the other hand, in systems with limited volume and mass, where it is impossible to change the specific volume, the situation looks different. Below we will look at the operating principle of such a system using a pressure cooker as an example. But let's return to our system, which allows for changes in specific volume. The specific volume in it will increase until all the liquid evaporates and the system again reaches equilibrium.
To design boilers and turbines used in power plants, such as those powered by natural gas, such as in the photo, an understanding of heat exchange and pressure changes in two-phase systems is necessary
We have just become acquainted with systems with constant pressure. Now consider a system with constant temperature and changing pressure. For each substance there is a range of pressures at which it can only exist in a gaseous state. There is also a range of pressures at which a substance can be both a liquid and a gas. It is worth noting that when the pressure changes, the specific volume also changes.
The threshold after which a substance cannot simultaneously be in two states of aggregation also exists for a liquid. The temperature threshold is called the critical temperature, and the pressure threshold is called the critical pressure. The combination of temperature and pressure at which the differences in the properties of the liquid and gaseous phases of a substance disappear is called the critical point in thermodynamics.
Physical properties of liquid
The main physical properties of a fluid that are considered in hydrodynamics include density, specific gravity, specific volume, thermal expansion, compressibility and viscosity.
Density is the ratio of the mass of a substance to its volume:
The density of a liquid is affected by temperature and pressure. The densities of some liquids are given below:
Specific gravity is the ratio of the weight of a liquid to its occupied volume:
Specific volume of a liquid is the volume of a unit mass of this liquid:
Thermal expansion is the property of a liquid to change its volume when the temperature changes. As the temperature increases, the volume of the liquid increases and vice versa. Different liquids increase in volume differently when the temperature increases by the same amount. Therefore, the property of a liquid to increase in volume with increasing temperature is characterized by the coefficient of thermal expansion Bt, which shows the change in unit volume of a given liquid when its temperature changes by 1 K.
The increase in volume upon heating is calculated using the equation:
delta V= Wt*V0*delta T
where V0 is the initial volume of liquid; delta T—temperature change.
In internal combustion engine calculations, the coefficient of thermal expansion is considered constant, although in fact it depends on the heating or cooling conditions, pressure and initial temperature.
Compressibility is the property of a liquid to change volume when pressure changes.
delta V = Bр*V0*delta р,
where delta V is the change in volume; delta p—pressure change; BP is the volumetric compression ratio.
The volumetric compression ratio shows the change in unit volume of liquid when pressure changes by 1 Pa. It depends on compression conditions, temperature and initial pressure. This dependence is not taken into account in the calculations.
The volumetric compression ratio for water is 5 * 10^-4 1/Pa, for oil products - 7 * 10^-4 1/Pa, for mercury - 0.3 * 10^-4 1/Pa.
Due to their insignificant values, liquids are considered incompressible.
Viscosity is the property of a liquid to resist the movement of one part of it relative to another.
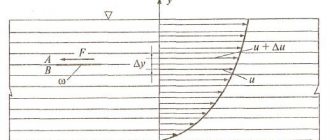
Rice. Scheme of changing the speed of a fluid enclosed between a fixed (1) and movable (2) plate
If plane 2, located at a distance b from plane 1, under the influence of force F moves with speed V0, then the layers of liquid located between the planes move at different speeds. In this case, the maximum speed V0 is at the points of contact with plane 2, the minimum (down to zero) at the points of contact with plane 1.
Temperature, pressure and specific volume
In thermodynamics, pressure, temperature and specific volume are three quantities that are interconnected and dependent on each other. Since these quantities are easy to find, they are convenient to use to describe thermodynamic systems. As we described above, if a substance is in one phase, then a change in pressure or a change in temperature causes an increase or decrease in the specific volume. How this specific volume changes depends on the substance, but for most gases, increasing pressure at constant temperature causes the specific volume to decrease. On the other hand, increasing the temperature at constant pressure most often increases the specific volume. This dependence also allows you to control pressure or temperature by changing the specific volume. This is exactly the principle that a pressure cooker works on.
The boiling point of water in a pressure cooker increases to 121 °C (250 °F) at sea level at a pressure that is 1 bar or approximately 15 psi above atmospheric pressure at sea level.
Densities and specific volumes of some substances[ | ]
The table shows densities and specific volumes for some substances. The values are given for standard temperature and pressure: 0 ° C (273.15 K) and 1 atm (101.325 kPa or 760 mm Hg).
| Substance name | Density, (kg/m3) | Specific volume, (m3/kg) | Substance name | Density, (kg/m3) | Specific volume, (m3/kg) |
| Air | 1.225 | 0,816 | Carbon dioxide | 1,977 | 0,506 |
| Water ice | 916,7 | 0,00109 | Chlorine | 2,994 | 0,334 |
| Liquid water | 1000 | 0,00100 | Hydrogen | 0,0899 | 11,12 |
| Sea water | 1030 | 0,00097 | Methane | 0,717 | 1,39 |
| Mercury | 13546 | 0,00007 | Nitrogen | 1,25 | 0,799 |
| Freon R-22* | 3,66 | 0,273 | Water vapor* | 0,804 | 1,24 |
| Ammonia | 0,769 | 1,30 | |||
| * — values are indicated for non-standard temperature and pressure | |||||