Hot galvanizing is the most reliable, most effective and at the same time quite old method of protecting products from corrosive influences. Hot-dip galvanizing allows for reliable protection of the surface of metal structures from the damaging effects of the environment. A protective layer is formed on the elements, which evenly covers the parts, and its thickness ranges on average from 30 to 120 microns. A horizontal bath 13 m long, 1.8 m wide and 2 m deep makes it possible to galvanize large diameter pipes, power line supports and various metal structures. The average service life of galvanized products, depending on the conditions of their use, is 25-30 years.
Due to hot galvanizing, the coating is provided not only with a barrier, but also with electrochemical protection of the metal from corrosion. Galvanizing, which can be performed using different technologies, is used mainly in relation to steel.
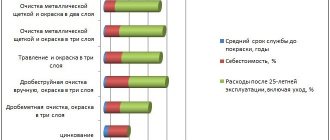
In terms of cost and durability, hot zinc coating is superior to other types of galvanizing, such as electrochemical galvanizing. Hot galvanizing of metal, when compared with other technologies, is characterized by an optimal combination of the affordable cost of the technological process with the high protective properties of the resulting zinc layer. The coating applied by hot-dip galvanizing, even when the product is used in the most unfavorable conditions, can last a long time, while fully retaining its protective properties.
The essence of galvanizing
With any technology, its implementation comes down to protecting the metal from corrosion. Depending on the technique used, the protective layer can wrap the surface of the material, or additionally connect with it due to diffuse interaction.
Due to the properties of zinc, upon contact with atmospheric oxygen, an oxide film is instantly formed on its surface, which has a high density and strength, due to which it does not allow atmospheric oxygen to pass through to the metal and is not destroyed by mechanical stress.
Thus, the galvanized metal ends up in a reliable airtight shell, protecting it from destructive corrosion.
In the event of mechanical impact, the oxide film is damaged, the exposed zinc immediately interacts with atmospheric oxygen, which forms a new protective layer. Due to this property, galvanizing is called self-healing metal protection against corrosion.
Metal galvanizing methods
There are several methods of galvanizing metal, which have their own characteristics. The chosen method significantly influences, to one degree or another, the result obtained, since, depending on the scope of use of metal products and structures, it is not economically profitable to use all galvanizing technologies.
Galvanizing methods differ in the following properties:
Thickness of protective coating
— if for large metal structures the larger the layer of protection, the better, then for high-precision small parts it is necessary to use galvanizing, in which you can select and control the thickness of the anti-corrosion coating. Not every technology allows this to be realized.
Uniform thickness of galvanizing
- again, the smaller and more precise the metal part, the higher the requirements for the protective coating applied to it. For example, for a power line support, it does not matter how evenly it is covered with zinc, while for parts with holes, threads and chamfers, the uniformity of the zinc layer is extremely important.
Strength of retention of the protective coating on metal
— this parameter strongly depends on the method by which galvanizing was performed. So, for example, with hot zinc discussed below, it not only covers the surface of the product, but also connects with it at the molecular level, which significantly increases the strength of the protection on the metal.
Appearance of a galvanized part
— depending on the technology used, the surface layer of zinc oxide can be matte, glossy, and also vary in shades from dark gray to bluish.
Resistant to mechanical damage
— the higher this parameter, the longer the protection will last on products that are subjected to certain physical stress and aggressive factors.
Self-healing ability
- this ability depends on the thickness of the zinc deposited on the metal and the nature of the operation of the structure or product.
Corrosion resistance
- is a general property of a processed product, which consists of a combination of several factors. In particular, the resistance of a galvanized part to corrosion depends on the thickness of the zinc layer, its uniformity, retention strength, as well as resistance to mechanical damage and self-healing ability.
In modern industry, the most common galvanizing technologies are:
Hot
- performed by immersing the workpiece in molten zinc.
Cold
- carried out in absolute analogy with conventional painting by hand or by spraying.
Galvanic
— is implemented by immersing the workpieces in a zinc-containing electrolyte through which an electric current is passed.
Thermal diffusion
— a layer of protective coating is formed when the workpiece is placed in an environment saturated with powdered zinc.
Gas-thermal
— a gas burner is directed to the workpiece and zinc wire or zinc powder is supplied to the spot of greatest heating.
Let's look at the main advantages, disadvantages and hot-dip galvanizing technology
Hot galvanizing is carried out in the following sequence
Degreasing
The procedure removes contaminants (for example, oil) from the surface of parts. It is carried out at temperatures from 60°C to 80°C using degreasing reagents, the choice of which is determined depending on the type of contamination. Degreasing prevents delamination of the zinc coating after its application.
Flushing
Removing foam and greasy compounds from the surface of products that settle on the metal after a degreasing bath.
Etching
Cleaning the surfaces of products by removing rust from them (when stored in unacceptable conditions) or scale (formed after hot processing). The operation is carried out at a temperature range from 20°C to 25°C using hydrochloric acid in a concentration of 120 – 210 g/l.
This ensures high solubility of ferric chlorides. To prevent hydrogen saturation and achieve removal of only hydroxyls and oxides from the surface, it is recommended to supplement the hydrochloric acid solution with inhibitors.
Repeated flushing
To neutralize residual traces of acid, as well as to remove salts, repeated washing of the parts is required. The use of several rinsing baths at once optimizes the rinsing process and at the same time reduces water consumption.
Fluxing
This is the final process of preparing the surface of parts on which iron oxides may have reappeared during washing. Fluxing prevents subsequent oxidation of the metal due to the formation of a passivated flux film on the surface, and also guarantees a high degree of wettability with molten zinc.
Treatment is carried out at a temperature of 60°C using a concentrated flux solution of 400 - 600 g/l, the composition of which includes ammonium chloride and zinc chloride.
Cleaning is done with the addition of hydrogen peroxide, which continually deposits ferric salts to the bottom of the bath. Subsequently, the sediment enters the settling and filtration system.
Preheating and drying
At this stage, residual moisture is removed from the surface of the products, which makes it possible to prevent the zinc from splashing out with water vapor when processing hollow elements when the parts are immersed in the oven and deformed.
The operation helps heat the metal up to 100°C, increasing the efficiency of the furnace, saving energy and reducing the cost of galvanizing.
Since drying takes longer than hot-dip galvanizing of metal, it is advisable to provide at least 2 chambers in the drying oven.
Galvanizing
The zinc alloy is applied to the metal surface at a temperature of 445°C to 460°C, which reduces the appearance of oxides, matte and other formations. The exhaust gases are extracted thanks to an aspiration and filtration system.
Hot galvanizing
Hot zinc coating is carried out in accordance with GOST 9.307-89 “EZSKS, Hot zinc coatings.
General requirements and control methods." Extract from GOST 9.307-89 (clause 2. Coating requirements): 2.1. Appearance of the coating
2.1.1. Upon external inspection, the surface of the zinc coating should be smooth or rough, the coating should be continuous. The color of the coating ranges from shiny silver to matte dark gray.
2.1.2. There should be no cracks, nicks, or swelling on the surface of the products.
2.1.3. The presence of zinc deposits is unacceptable if they interfere with assembly. Grains of hartzink with a diameter of no more than 2 mm, surface rippling, light gray spots and tarnished colors, scratches, scratches, traces of being caught by lifting devices without coating to the base metal are not defects.
Restoration of uncovered areas is acceptable if they are no wider than 2 cm and constitute no more than 2% of the total surface area. Uncoated areas are protected with a layer of zinc-containing paint coating (minimum thickness 90 microns, mass fraction of zinc in the dry film 80%-85%) or thermal spraying of zinc (minimum thickness 120 microns).
Features and advantages of hot-dip galvanizing
The mechanism for protecting galvanized steel structures from corrosion is that the zinc coating is anodic, while the metal underneath is the cathode, and therefore is not subject to corrosion. Zinc serves as a protective layer as long as it is present on the metal and is not damaged, but this coating is durable and can last for decades.
Hot-dip galvanizing is the best way to protect cast iron and steel from corrosion, for both economic and environmental reasons.
Advantages of hot galvanizing:
- Corrosion resistance. It is ten times higher than with electrochemical coating.
- Self-healing coating. Due to the fact that zinc is highly anodic, it independently restores damaged parts of parts.
- Increased strength. The coating increases the service life of the steel by another fifty years. Hot-dip galvanized steel does not expand or contract with changes in humidity.
- Impact resistance. The steel hardness is 160 on the Vickers scale. The zinc coating has a hardness of 240 degrees. When impacted, the zinc coating absorbs the force, preventing it from affecting the base material. Therefore, only the galvanization may be damaged and not the steel itself. In addition, zinc, a ductile material, provides shock absorption and strong shock absorption.
- Creates freedom to create designs. Galvanized coating looks aesthetically pleasing. It has a characteristic shiny structure that makes it attractive. In many modern buildings, galvanized balustrades and handrails are decorative elements that reflect the modernity of the building. But this does not mean that galvanized steel should remain in its original form. The coating allows you to paint with paint of any color. If there is a need to match the color of a galvanized steel element to the color of the interior or environment, this is not a problem.
- Economical coverage. Elements galvanized by this method do not require repainting during operation.
Features of hot zinc coating
On steels with a high content of silicon and/or phosphorus (due to the heterogeneity of the chemical composition of the steel), (layers near the surface, surface structure, foreign inclusions), as well as on thick-walled products, different shades of coating are observed. The gray surface consists of a zinc-iron alloy exposed on the surface, while the lighter areas are pure zinc. In the case of steel with an unfavorable content of silicon and phosphorus, a significant thickening of the coating and a deterioration in its adhesion may occur; the visible effect of this phenomenon may be a rough surface with a gray and dark gray color, turning over time even into a brown tint.
The zinc surface on the same product may not be uniform; spots of varying degrees of gloss, gray dullness and roughness may appear. Since the surface localization of impurities in low-alloy steel leads to the fact that when rolling products from it, a significant difference appears in the content of silicon and phosphorus in different parts of the product.
However, this cannot be a defective sign, since, apart from the appearance, there are no negative aspects - it does not affect the service life of the coating, and after some time (within 1-1.5 years) the color of the structure will become the same).
White corrosion (white-gray spots that appear under the influence of atmospheric factors), as well as so-called “bloody streaks” (flux and acid residues in places of loose welding) are not grounds for claims about the quality of the galvanized surface.
The above problems are most typical for steel grade 09G2S, which is due to the peculiarities of their chemical composition.
The most common defects in the zinc coating for 09G2S steels, which are not grounds for filing claims:
1. Increased thickness of the resulting zinc coating.
2. Different thickness of zinc coating.
3. Increased formation of hard zinc.
4. Reduced adhesion of the zinc coating to the metal.
5. Reduced density of the zinc layer.
6. Appearance of the coating (lack of shine, unevenness, streaks, gray color).
Zinc hardness
The strength of any paint coating is determined by the strength of the binder.
Varnishes by hardness, in accordance with international standards ISO 15184, ASTM D 3363, SIS 184187, NEN 5350, ECCA Test Method, are determined using pencil leads on a scale of 6B-5B-4B-3B-B-HB-F-H-2H -3H-4H-5H-6H-7Н-8Н-9Н, where 6B is the lowest hardness and 9H is the highest hardness. It has the same hardness as a 2T pencil.
The hardness of hot-dip galvanizing is equal to the hardness of the metal. Zinc. And under zinc we have iron.
According to the Mohs jewelry scale:
Talc (graphite) - 1
Lead - 1.5 Amber (gypsum) - 2 Zinc (aluminum, gold, silver) - 2.5-3 Copper - 3 Iron - 4 Steel - 5 Glass - 6 ... Diamond - 10
Equipment used – hot-dip galvanizing line
In the mass production of parts and protective coatings of sheet metal, wire and other metal products, hot-dip galvanizing equipment is installed in one line, along one axis. The length of the workshop reaches several hundred meters. On top are overhead cranes that have a protective coating against exposure to chemicals.
Chemical preparation area
Several baths stand in one line in accordance with technological operations. The first is degreasing, then etching. The final items after fluxing are containers with water for rinsing. The drying chamber completes the chemical preparation.
The traverse with boxes or metal structures is fed to the first container on a trolley. Then the tap lowers it into all the baths one by one. For large tank sizes, it is possible to transfer parts between tanks via a conveyor.
Hot dip galvanizing furnace
A furnace for continuous hot-dip galvanizing is designed in length to smoothly lower the baskets and move them to the opposite end based on the speed of dipping and removing, and the heating time of the steel. The average bathtub length is 13 m.
Continuous sheet galvanizing line
Heating is carried out in different ways. Gas is recognized as the most profitable. The bath can be heated with electricity, fuel oil and coal. It all depends on nearby energy sources.
Hot-dip galvanizing protects metal structures from corrosion. The method has its drawbacks, but there are many more advantages. The coating method is relatively cheap and fast.
We are interested in readers' opinions regarding hot-dip galvanizing. Perhaps other technologies for coating metal with zinc are more convenient and practical?
Technological cycle of hot-dip galvanizing
A complete hot-dip galvanizing process includes the following technological steps:
Loading of products requiring processing to the hanging area
. Since the method under consideration is one of the few that allows galvanizing relatively large parts, shipment is usually carried out using special overhead cranes.
Hanging metal structures
. For subsequent processing, parts delivered to production are hung on movable crossheads. Metal structures are distributed and fixed in such a way that the entire section can fit into technological containers at further stages. It is also important to hang the products so that they can come into contact with the liquids in which they will be immersed over the entire surface, without interfering with each other.
Pre-treatment of metal structures.
Before hot galvanizing, steel products undergo mandatory multi-stage preparation. It consists of alternately immersing a traverse with suspended parts in baths with process fluids. Including, in these baths, degreasing, cleaning, etching (which ensures the penetration of zinc into the crystal lattice of the metal), removal of traces of acid, and coating with protective flux are carried out. Also at this stage, the metal is preheated before immersion in molten zinc, which avoids deformation of products due to sudden temperature changes.
Drying and preheating
. It is carried out in a special multi-stage oven into which heated and purified air is supplied. As a result, before galvanizing, traces of preliminary preparation in baths of liquids evaporate from metal structures, and they are also heated additionally.
Galvanizing
. The main technological stage of the hot-dip galvanizing process. It is carried out by transporting a traverse with prepared metal products into a furnace closed on all sides, in which a bath of molten zinc is located. Its temperature is maintained at a constant level around +450°C using high-speed gas burners. The sealing of the furnace is necessary for two reasons.
Firstly, this is necessary to ensure the safety of people who work in production. Secondly, during the galvanizing process, gases heated to a high temperature are released, which need to be purified before being released into the atmosphere. In addition, the thermal energy of these gases is used again to heat process fluids at the stage of preliminary preparation of metal products.
Removal, sorting and shipment of galvanized metal structures
. Upon completion of the galvanizing process in a bath of molten zinc, the traverse with hanging products is automatically sent to the area for their removal and sorting, after which the metal structures are loaded onto transport for shipment to the customer.
Despite the apparent complexity of the described process, the hot-dip galvanizing method is one of the simplest and most cost-effective. In addition, thanks to the introduction of certain technological stages, it is possible to ultimately obtain anti-corrosion protection with numerous advantages compared to other galvanizing methods.
Hot-dip galvanizing. Technological characteristics of the process
Hot-dip galvanizing is determined by the rate of the diffusion process, which directly depends on • the temperature regime, • the state of aggregation of the interacting substances.
The density of the surface layer is 7.13 g/cm3.
The microhardness of the zinc layer after hot-dip galvanizing is 360 MPa. Hot galvanizing occurs by briefly immersing a metal product in molten zinc (working solution temperature ~ 450 degrees, holding time - 9-12 minutes).
The hardness and wear resistance of the coating is increased due to the formation of a protective layer consisting of zinc carbonate on the surface. When the workpiece is removed from the molten solution, a series of sequential chemical reactions occur. First, zinc oxide is formed (zinc reacts with oxygen and oxidizes). Then, as a result of the interaction of carbon dioxide and zinc oxide, a stable, solid compound is obtained - zinc carbonate.
The zinc coating after finishing has a gray, matte color. To improve the decorative characteristics, aluminum and nickel are added to the zinc melt (together or separately).
After hot-dip galvanizing, the applied coating layer can be in the range from 30 to 200 microns. The thickness of the zinc layer depends on • the operating temperature of the molten solution, • the immersion time interval, • the speed mode for removing the workpiece from the galvanizing bath, • the final removal of excess zinc melt.
The high wear resistance of the zinc coating and the large thickness of the protective layer increase the service life of the processed products.
On average, metal structures after hot galvanizing last 60 years or more.