Types of corrosion
Methods for protecting metals from corrosion are selected depending on the operating conditions of the products. Therefore it stands out:
- Corrosion associated with atmospheric phenomena. This is a destructive process of oxygen or hydrogen depolarization of a metal. Which leads to the destruction of the crystalline molecular lattice under the influence of a humid air environment and other aggressive factors and impurities (temperature, the presence of chemical impurities, etc.).
- Corrosion in water, primarily sea water. In it, the process goes faster due to the content of salts and microorganisms.
- Destruction processes that occur in the soil. Soil corrosion is a rather complex form of metal damage. Much depends on the composition of the soil, humidity, heating and other factors. In addition, products, for example, pipelines, are buried deep in the ground, which makes diagnostics difficult. And corrosion often affects individual parts pointwise or in the form of ulcerative veins.
Types of corrosion protection are selected individually, depending on the environment in which the metal product being protected will be located.
Fighting corrosion in all available ways
Every car owner who cares about his car should know about the ways that help prevent the development of this unpleasant factor. It is worth noting that among modern drugs you can even find substances that allow you to avoid the manifestations of corrosion of both atmospheric and mechanical types.
One of the very first methods is passive exposure, which involves isolating metal parts and the body from the influence of atmospheric air. Another effective method is active action aimed at creating a special film that protects the metal. As a result of this effect, the car body gets rid of the oxidized layer, and the soil that appears on the vehicle parts repels liquid, salt and acid, thereby neutralizing all substances that can affect the further development of corrosion.
There are also mastics on sale, which are passive substances that reliably protect the underbody of the car. They are based on rubber, resin and bitumen. Oil and graphite are present as active additives; fibrous materials are no exception. After purchasing, the mastic must be applied in a thick layer to the bottom of the vehicle. In order for the maximum effect to be achieved, the car must be prepared by first treating the slot holes with a special anti-corrosion substance that prevents the penetration of other negatively affecting substances.
Typical types of rust damage
Methods for protecting steel and alloys depend not only on the type of corrosion, but also on the type of destruction:
- Rust covers the surface of the product in a continuous layer or in separate areas.
- It appears in the form of spots and penetrates pointwise into the depths of the part.
- Destroys the metal molecular lattice in the form of a deep crack.
- In a steel product consisting of alloys, destruction of one of the metals occurs.
- Deeper extensive rusting, when not only the surface is gradually damaged, but also penetration occurs into the deeper layers of the structure.
The types of damage can be combined. Sometimes they are difficult to determine immediately, especially when point destruction of steel occurs. Corrosion protection methods include special diagnostics to determine the extent of damage.
They produce chemical corrosion without generating electrical currents. Upon contact with petroleum products, alcohol solutions and other aggressive ingredients, a chemical reaction occurs, accompanied by gas emissions and high temperature.
Galvanic corrosion is when a metal surface comes into contact with an electrolyte, specifically water from the environment. In this case, diffusion of metals occurs. Under the influence of the electrolyte, an electric current arises, the replacement and movement of electrons of the metals that are included in the alloy occurs. The structure is destroyed and rust forms.
Steelmaking and its corrosion protection are two sides of the same coin. Corrosion causes enormous damage to industrial and commercial buildings. In cases with large-scale technical structures, for example, bridges, power poles, barrier structures, it can also provoke man-made disasters.
Corrosion
Metal corrosion is a natural phenomenon that cannot be completely prevented, but this destructive process can be significantly slowed down. The oxidation process occurs with the participation of oxygen and aqueous solutions containing acid, alkali or salt.
Iron is not found in nature in its pure form, but is found in iron ore. Humanity invented the production of steel and came up with ways to preserve it. Factories use methods of phosphating steel by immersing it in various solutions, as well as electrochemical treatment. This coating is in the nature of a primer and requires subsequent painting. Steel is coated with other metals. Cheaper ones are aluminum and zinc.
There are silicate coatings - these are different types of enamel. Enamel is fragile and not entirely suitable for a fence. Cement has approximately the same expansion temperature as steel and serves as an insulator against aggressive environments. Good insulation is a polymer film applied in several layers in the factory.
READ ALSO: How to Place Fence Posts in a Swamp
Metal corrosion and methods of protection against it
How to protect metal? There are many corrosion methods for metals and ways to protect against it. To protect metal from rust, industrial methods are used. In everyday life, various silicone enamels, varnishes, paints, and polymer materials are used.
Industrial
Protection of iron from corrosion can be divided into several main areas. Methods of protection against corrosion:
- Passivation. When producing steel, other metals are added (chromium, nickel, molybdenum, niobium and others). They are distinguished by increased quality characteristics, refractoriness, resistance to aggressive environments, etc. As a result, an oxide film is formed. These types of steel are called alloyed.
- Surface coating with other metals. Various methods are used to protect metals from corrosion: electroplating, immersion in a molten composition, application to the surface using special equipment. As a result, a metal protective film is formed. Chromium, nickel, cobalt, aluminum and others are most often used for these purposes. Alloys (bronze, brass) are also used.
- The use of metal anodes, protectors, often made of magnesium alloys, zinc or aluminum. As a result of contact with the electrolyte (water), an electrochemical reaction begins. The protector breaks down and forms a protective film on the surface of the steel. This technique has proven itself well for underwater parts of ships and offshore drilling rigs.
- Acid etching inhibitors. The use of substances that reduce the level of environmental impact on metal. They are used for preservation and storage of products. And also in the oil refining industry.
- Corrosion and protection of metals, bimetals (cladding). This is coating steel with a layer of another metal or a composite composition. Under the influence of pressure and high temperatures, diffusion and bonding of surfaces occurs. For example, well-known heating radiators made of bimetal.
Metal corrosion and methods of protection against it used in industrial production are quite diverse, such as chemical protection, glass enamel coating, and enameled products. Steel is hardened at high temperatures, over 1000 degrees.
On video: galvanizing metal as protection against corrosion.
Household
Protecting metals from corrosion at home is, first of all, chemicals for the production of paints and varnishes. The protective properties of the compositions are achieved by combining various components: silicone resins, polymer materials, inhibitors, metal powder and shavings.
To protect the surface from rust, it is necessary to use special primers or a rust converter before painting, especially old structures.
What types of converters are there:
- Primers - provide adhesion, adhesion to metal, level the surface before painting. Most of them contain inhibitors that significantly slow down the corrosion process. Preliminary application of a primer layer can significantly save paint.
- Chemical compounds - convert iron oxide into other compounds. They are not subject to rust. They are called stabilizers.
- Compounds that convert rust into salts.
- Resins and oils that bind and seal rust, thereby neutralizing it.
These products contain components that slow down the process of rust formation as much as possible. Converters are included in the product line of manufacturers producing metal paints. They vary in consistency.
It is better to choose primer and paint from the same company so that they match the chemical composition. You must first decide which methods you will choose to apply the composition.
Protection of fire equipment from corrosion
Parts of PA mechanisms and systems are in contact with the external environment, engine exhaust gases, fire extinguishing agents, and operating materials. The metals of parts and systems are not always neutral relative to each other. For this reason, an irreversible change in the state of metal surfaces and their destruction may occur. The destruction of metals under the influence of the environments with which they are in contact is called corrosion.
. Depending on the medium causing corrosion, it can be liquid, gas, or atmospheric. However, according to the mechanism of corrosion processes, a distinction is made between chemical and electrochemical corrosion.
Chemical corrosion occurs when a metal reacts chemically, for example, with atmospheric oxygen or corrosive substances contained in liquids that do not conduct electrical current. An example is the corrosion of parts of diesel fuel supply equipment (Fig. 15.4). It is caused by mercaptans (R-H-S) contained in fuel. They are very aggressive even in the presence of traces of moisture.
Rice. 15.4. Corrosion of working surfaces of diesel fuel supply equipment parts:
A
– fuel pump rack:
1
– rack;
2
– corrosion;
b
– regulator parts:
1
– plate;
2
– ball;
3
– corrosion
Diesel cylinder liners and their piston rings are subject to chemical corrosion. It is caused by condensing exhaust gases.
Diesel fuel contains up to 0.2% of various compositions of sulfur-containing substances. When fuel burns, depending on the operating mode of the engine, sulfur oxides SO2 or SO3 are formed, contained in the exhaust gases. In the presence of moisture, the oxides are converted into acids H2SO3 or H2SO4. They chemically interact with the metal of the cartridge cases, exposing them to corrosion. If special measures are not taken during storage (preservation), then after a certain period of corrosion during subsequent operation of the PM will lead to an increase in wear of cylinder liners by 1.5-2 times, and of the crankshaft journals by 15-20%.
Electrochemical corrosion occurs when chemical corrosion is accompanied by the flow of electric current on the surface of the metal and the environment. Electrochemical corrosion occurs if the medium interacting with the metal is an electrolyte. Its role can be played by moisture adsorbed on metal surfaces. It may contain oxides, for example, SO2, CO2, sea salts near the seashores.
The source of electric current is the difference in electrode potentials of different metal particles. Let, for example, a fragment of a metal part contain Zn, Cu and Fe (Fig. 15.5). Their electronic potentials are respectively –0.76; +0.34 and –0.23 V. Under a drop of moisture, which is an electrolyte, three microgalvanic cells are formed. In addition, each of them forms a microvoltaic cell with steel (-0.36 V). Particles with a more negative electrode potential will be destroyed.
On a metal surface unprotected from the influence of the external environment, countless such microelements are formed. Often this type of corrosion occurs in an air atmosphere, which is why it is called atmospheric corrosion.
In real conditions, metals are heterogeneous. On the surface of metal products there are crystalline grains of various orientations, the metal composition of which may be different due to microliquation; the alloy itself may have a heterogeneous structure. Because of this inevitable heterogeneity, different parts of the surface of parts are characterized by different potentials. Areas with a more negative electrode potential play the role of anodes. They will be destroyed. Mechanical stress also increases negative potentials, they enhance electrochemical corrosion.
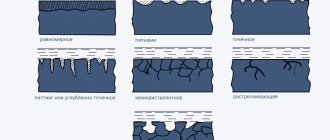
The heterogeneity of the metal of the parts, different stresses in their different parts, and the unequal intensity of corrosion processes are the cause of the formation of various forms of corrosion lesions (Fig. 15.6).
Rice. 15.6. Forms of corrosion damage:
1
– continuous uniform;
2
– continuous uneven;
3
– spots;
4
– ulcers;
5
– point;
6
– intercrystalline
As a result of corrosion, oxide films are formed on metal surfaces. Films on steel are loose, fragile, and easily destroyed. This process is continuous and causes the destruction of metals. During the year, about 20 million tons of metal structures fail in our country.
The oxide layer on parts made of non-ferrous metals is strong, dense, and protects the metal from corrosive destruction. Such products do not require corrosion protection.
The intensity of corrosion is largely determined by the properties of the environment affecting the metals.
Characteristics of corrosive environments. Fire extinguishing agents (water, foaming agents) wash the surfaces of water-foam communications elements. Various gases and salts are dissolved in water, so it is a weak electrolyte. The inner surface of the tank above the water level is wetted by its vapors, and they condense on it. Corrosion can also occur on the surfaces of tanks filled with liquid. To prevent it, paint and varnish materials and anodic protection are used.
Foaming agents are corrosive. Therefore, the tanks for them are made of stainless steel. During operation, after using foam concentrates, it is necessary to flush the foam supply system.
Drops of foaming agent entering the pump compartment cause severe corrosion of threaded connections. This makes dismantling the pump difficult.
Operating materials. Lubricants, shock-absorbing fluids and lubricants do not cause corrosion.
Sulfur-containing substances in fuel can cause corrosion of fuel supply equipment, and combustion products can cause corrosion of cylinder liners. To prevent it, special measures must be taken.
Atmosphere. The gas composition of the air at the surface of the earth is relatively constant. The content of moisture, industrial gases, and dust is variable.
The degree of dryness of the atmosphere is measured by relative humidity φ, %.
It is equal to the ratio of the absolute (real) moisture content
a
, g/m3, to its maximum possible content
A
, g/m3, at a given temperature
. (15.12)
Pure moisture causes virtually no corrosion (direct 1
in Fig.
15.7). In the presence of SO2 (curve 2
), corrosion increases greatly.
However, at R
< 66% it practically stops. This is due to the fact that dew does not fall during any possible fluctuations in air temperature.
Rice. 15.7. The influence of atmospheric pollution on steel corrosion:
1
– pure moisture;
2
– moisture and 0.05% SO2;
3
– the same, samples covered with SiO2 dust
Dust has a great influence on corrosion (curve 3
), since due to the crevice (capillary) effect, moisture condenses under the anthers.
Corrosion protection. The various states of metal surfaces and the composition of the media in contact with them determine different mechanisms of corrosion. Naturally, this requires various methods of protection against it.
Let's analyze them in general terms. The amount of electric current flowing in a microvoltaic cell, μA, is determined by the ratio of the potential difference of the elements to the resistance of the medium
. (15.13)
In production, they strive to select metals so that εа —
εк
®
0. In operation, conditions are created to isolate products from the influence of the external environment or increase additional resistance
R
add.
In general terms, methods for protecting metal surfaces and products made from them can be presented as shown in Fig. 15.8.
Rice. 15.8. Corrosion protection methods
Metal coatings are used in production. Products can be nickel-plated, zinc-plated or coated with other metals. These coatings are effective as long as the integrity of the coating layer is not compromised. Violating it will destroy metal that has a more negative potential. So, in the case of tin coating, the tin layer will be destroyed, and with galvanized surfaces, the zinc layer will be destroyed.
Non-metallic coatings – protection with oils, lubricants, paints (enamels). Liquid oils drain from vertical and inclined surfaces. The restoration of the lubricating protective layer is carried out by putting the mechanism into operation or by turning the mechanism shafts manually.
Greases, enamels or lubricating oils reliably isolate metal surfaces from the external environment. However, their protection is effective as long as the protective layer is not broken.
Protection using enamels (paints and varnishes) is quite effective. The structure of these coatings is complex (Fig. 15.9).
When it is destroyed, for example by a blow from a heavy object or a scratch, corrosion is not outwardly noticeable. It develops under the enamel layer (Fig. 15.10). This is due to the fact that the oxidizing purified metal has a more positive electrode potential. Therefore, it is necessary to expand the damaged layer, remove the resulting traces of corrosion and completely restore the painted layer. The same applies when restoring the protective layer with lubricants.
Treatment of corrosive environments is widely used in technology. Thus, antioxidant and anti-corrosion additives are introduced into lubricants. Coolants also contain various additives. Thus, 0.05% of champin (K2Cr2O7), sodium nitrite (NaNO2) and trisodium phosphate (Na3PO4) are added to the water for engine cooling systems. In addition, they prevent scale deposits in engine cooling systems.
Treatment of a corrosive environment also includes such a technique as removing exhaust gas residues from the combustion chambers of engines. To do this, use the starter to crank the engine crankshaft without fuel supply for 3-5 s.
An important technique for treating a corrosive environment is to create conditions under which the relative humidity in the object will not exceed 60%. Such conditions are created when products are packaged in sealed polyethylene shells, inside which silica gel (SiO23H2O) is placed. It absorbs moisture entering the packaging from outside, automatically maintaining humidity below 60%.
Preservation of fire trucks is carried out to prevent corrosion of mechanisms and systems of vehicles that will not be used for a long time.
Conservation means the maintenance of technically serviceable, fully equipped, fueled and specially prepared vehicles and equipment in a condition that ensures their long-term preservation and bringing them into combat readiness in the shortest possible time.
All supernumerary PAs or PAs, the use of which is not expected for a period of more than three months, and in special climatic conditions - more than one month, are put on conservation.
Conservation can be short-term (up to one year) and long-term - more than one year.
The placement of the PA for conservation is carried out by decision of the head of the State PS. It also determines the type of conservation, the list of machines subject to conservation, and the financially responsible persons for performing the work.
The conservation work is organized by the deputy head of the detachment (unit). He also draws up a work plan. The plan defines:
training of personnel who will perform the work;
distribution of equipment and premises for storing machines;
providing units with operational materials;
procedure for completing documentation.
A record is made in the form regarding the placement and removal of the PA from conservation.
The installation and removal from conservation of other equipment is carried out by the decision of the head of the GPS unit.
Conservation of PA. Only vehicles that have a range of at least 12,000 km before medium and major repairs are put into storage. New cars are put into storage after running-in.
Preparing machines for preservation includes regular maintenance and special work to protect them from corrosion. Before starting special work, fire hoses, hose delays, rescue ropes, tools for cutting electrical wires, and portable electric lights are removed from the vehicles and stored separately. The rest of the fire-technical equipment, after bringing it into serviceable condition, is lubricated if necessary and stored on the machines.
Additional work to protect mechanisms from corrosion is regulated by the Technical Service Manual. They can be divided into two groups. First group
consist of work performed before the vehicles are placed at the parking lot for storage.
The second group
consists of work after installing cars in parking lots for storage.
The works of the first group include:
washing, cleaning all tanks and drying them, and, if necessary, restoring damaged layers of paint;
the wheels are removed from the cars, the tires are dismantled, cleaned of corrosion, the tires are painted, the tubes are soaped*, then the tires are mounted on rims, filled with air and installed in place;
removing water from the working cavity of the pump and pouring a liter of engine oil into this cavity; turning the pump shaft 5-10 turns, drain the oil; the foam mixer is removed, disassembled, after cleaning, lubricated with engine oil and installed in place;
treatment with grease of all drive hinges, tools, spring leaves (graphite lubricant).
Upon completion of all work, all gates and valves are closed, and a plug is placed on the suction pipe. After completing this work, the car is placed in its parking place. Special premises are allocated for storing cars. In them, cars can be installed in no more than two rows with their front part facing the gate with an interval of at least 1 meter.
The work of the second group includes engine preservation, draining water and fuel from the engine. The most difficult thing is engine preservation.
Warming up the engine before preservation should be carried out at operating speeds at a coolant temperature of 70-80 °C. In this case, the smallest amount of SO2 and SO3 is formed in the exhaust gases.
Stop the engine by turning off the petrol valve in the fuel tank. Having turned out the spark plugs, you should turn the crankshaft (2-3 times) with the starter for 5 s. After this, 30-50 g of dehydrated (preheated to 110 ° C) oil at 80 ° C is poured into each cylinder, then the engine crankshaft is turned by the crank 10-15 turns. The spark plugs are screwed into place and all operating materials are drained from the engine. Batteries are removed from vehicles, prepared for storage and delivered to the warehouse. The necks of all mechanisms and systems are sealed and covered with oiled paper.
The controls should be in neutral and the brakes released.
Each car is placed on metal or wooden stands. In this case, the wheels should be raised from the ground by 8-10 cm, and the air pressure in the tires should be reduced by 50%.
After the commission has checked the quality of conservation, the cabin and body doors, as well as the engine hood, are sealed.
The ignition keys must be in place.
Maintenance of vehicles during conservation is carried out according to inspection plans. During maintenance, a complex of works is performed. First of all, check for corrosion. If it is detected, it is removed, followed by covering the areas with grease. The operation of the control drives is checked. Upon completion of all work, the engine hood, cab and body doors are sealed.
Every year, 20% of cars are removed from storage. They are tested with a mileage of 20-25 km, and special units - within 1 hour. If any faults are found, then other vehicles can be tested.
After the tests, the machines are serviced in the scope of the second technical maintenance and put into storage. A record of the work performed is made in the form.
Removing cars from preservation. Before placing vehicles into combat crews, they undergo the first technical maintenance. In addition, the equipment removed from them is installed on the machines, all systems are washed, topped up and the oil in the units is replaced. The tanks are filled with foaming agent, the tank with water. The operation of the pump is checked by drawing water from an open reservoir.
* Mark of conformity is a designation used to inform consumers about the compliance of the certification object with the requirements of the St. Petersburg SD or the national standard
* Waterline – the line along the side to which the ship is immersed in water during draft.
* At some factories they are designated “Rapid Response Vehicles” - ABR.
* Talc – mineral 3MgO 4SiO2H2O, spec. weight 2.7–2.8; hardness 1 (on the Mohs scale, on this scale the hardness of a diamond is 10).
Protective paints for metal
Metal paints are divided into heat-resistant, which can be used at high temperatures, and for normal temperatures up to eighty degrees. The following main types of metal paints are used: alkyd, acrylic, epoxy paints. There are special anti-corrosion paints. They are two- or three-component. They are mixed immediately before use.
Advantages of paintwork for metal surfaces:
- protect surfaces well from temperature changes and atmospheric fluctuations;
- can be applied quite easily in different ways (brush, roller, spray gun);
- most of them are quick-drying;
- wide range of colors;
- long service life.
Hammer paints have proven themselves well; they not only reliably protect the product, but also create an additional decorative effect. Rusting metal becomes as good as new.
Of the inexpensive means available, you can use ordinary silverware. It contains aluminum powder, which creates a protective film on the surface.
Two-component epoxy compounds are suitable for protecting metal surfaces that are subject to increased mechanical stress, in particular the underbody of cars.
Primer
@gidpokraske.ru
This is an emulsion that is designed to break down rust and prevent further destruction of the metal and, as an element, binds the metal with subsequent painting.
Main types
- ferrous metal primer;
- non-ferrous metal;
- primer is a rust converter.
All types of primer have a component that breaks down rust, so an additional special primer is used only in severe cases of metal damage.
Varieties for ferrous metal
Paint on any surface will last longer and better if the surface is pre-treated with a primer. The primer creates a film between the surface and the paint, preventing the destructive action of oxygen, which causes corrosion of metal and rotting of wood.
For different surfaces and purposes, the primer is divided into types according to its composition and effect on the treatment surface.
Types of primer for metal
Insulating
@lkmprom.ru
Alkyd primer GF-021 and epoxy primer EP-0010, based on zinc white, red lead and various fillers. Protects metal from oxygen and moisture.
Phosphating
Two-component primer brand VL-02, VL-023, consisting of a base and phosphoric acid diluent.
Passivating
Brand FL-03K, GF-0119, significantly slows down corrosion. It contains pigments that dissolve in water entering through cracks, creating a protective film.
Protective
Primer grades XC-068, EP-057, EP-0284 consist of powders of zinc metal components with magnesium or lead, interacting with the electrolyte of the paint and varnish composition, the primer creates a film impenetrable to corrosion.
Inhibitory
Primer brand – EP-0180 combines the advantages of primer and enamel, increasing the protective power. Currently the most popular.
Metal protection at home
Corrosion and methods of protecting against it at home require compliance with a certain sequence:
1. Before applying a primer or rust converter, the surface is thoroughly cleaned of dirt, oil stains, and rust. Use metal brushes or special attachments for grinders.
2. Then apply a primer layer, allow it to soak in and dry.
3. Next, the product is painted in two layers. First let the first one dry. When working, you must use protective equipment (gloves, goggles, respirator).
Protecting metals from corrosion is a complex process. It begins at the stage of steel smelting. It is difficult to list all the methods for combating rust, since they are constantly being improved, not only in industry, but also for domestic use. Manufacturers of paint and varnish products are constantly improving their compositions, increasing their corrosion properties. All this significantly extends the service life of metal structures and steel products.
How to protect metal from corrosion at home (2 videos)
Various protective compounds (32 photos)
Electrochemical protection method
Electrochemical protection is a special protection that belongs to a variety of cathodic methods. Its composition is a pair of galvanic zinc metals maintained under voltage. This feature causes a certain effect to appear that helps reduce the occurrence of corrosion by 5 times.
Despite the seeming fabulousness of the method, its effectiveness has been proven by a number of complex tests conducted by more than one group of modern scientists. It should be noted that such protection has been used for quite a long time to maintain pipelines and metal structures in working condition.
Electrochemical protection allows the car owner to take care of his vehicle, protecting the most inaccessible places from corrosion. The reliable substance contains an electrical unit and a zinc plate. Which necessitates mandatory grounding and connection to the car battery. This method is especially common among residents of Europe and the USA; the product arrived in Russia relatively recently, but this did not prevent it from achieving good results.
Shapes of metal pillars
Fence posts can have a wide variety of configurations, from simple to designer:
- round;
- square;
- rectangular;
- screw;
- homemade.
Round pipes are the easiest and cheapest to purchase. The choice of their diameter depends on the design of the fence, and most often the size used is from 57 mm to 108 mm; in exclusive versions, the diameter is increased to 159 mm. The thickness is selected taking into account the characteristics of the material being filled in the spans: from 1.5 mm to 4 mm. The thicker, the longer the service life.
A good option is with drill pipes with a wall thickness of 5 mm.
Transverse logs are attached directly to the pipes by welding, or guides for fastening are welded onto the pipes. The guides can be made in advance by welding them to a clamp, which is put on the pipe and tightened with a bolt. With this installation option, an insulating pad made of cotton material or a special plastic lining suitable for the diameter of the pipe is placed under the clamp.
READ ALSO: How to insulate cracks in garage doors
Profiled posts have the shape of a square or rectangle. This form makes it possible to fasten the logs not only by welding, but also by using bolts or rivets. It is more convenient to work with them if the guide logs are made of wood or the fence is built in the “Ranch” style.
Screw posts are a pipe with a drill welded at its end. This option is used for quick installation of the fence, because there is no need to dig a hole for the post first.
Homemade poles are made from used material that is available (orphan), or from material that can be obtained. For example, iron corners are suitable.