Copper patination, or oxidation, is the creation of an artificial oxide film on the surface of the metal. It is used for aging copper and brass jewelry, bronze interior items (for example, parts of boxes or furniture, figurines). Antique dealers treat coins to give them an authentic appearance after cleaning when the natural patina has been damaged or had to be removed.
The composition of artificial oxide films is similar to atmospheric patina, but some of them are easily erased, so they are coated with varnish. There are several ways to process metal to apply an oxide film to it, and patination of brass and copper is carried out in the same way:
- Chemical (treatment with chemical reagents). Some solutions are toxic or working with them requires complex technology; others can be used at home without fear.
- Electrochemical (processing in a bath with electrolyte).
- Thermal (exposure to fire).
Patinated copper
Before starting work, the item is cleaned, degreased with alcohol or dish soap, washed and dried. Products with relief details and coins after processing are not wiped with a cloth, but dried in sawdust. The fabric erases fragile films, and moisture remains in the recesses, and the color in them continues to change, although less intensely.
Quick ways
1) Patination of copper at home can be done in all ways, but the simplest of them is thermal. If you run a stream of fire over the surface of a metal, you get rainbow stains from pale yellow and orange to purple - as the metal heats up, the color of the metal becomes darker. If you don't have a jewelry torch, hold the jewelry in pliers and "dip" it into the edge of the flame on the stove burner. Dip the hot part into water, and the “tarnished colors” will be fixed, and so that the color does not rub off, it is coated with varnish.
2) For simple chemical patination, use sulfur ointment. Apply a layer of ointment to the part and leave it for 3-4 hours, and then wash it with soap or dish soap.
- Sulfur ointment is very greasy, so do not use it on objects with relief: it is difficult to remove its remnants from the recesses.
- When copper oxidizes in air, it forms a dark, abrasion-resistant patina. Apply the composition to objects immersed in water, smearing them with Vaseline: the ointment will lie more evenly and the shade will be weaker.
- To remove an unsuccessful patina, place the jewelry or coin in a warm solution of citric acid for 10 minutes or wash with laundry soap under running water.
3) A safe and effective way to enhance the color of a brooch, ring, or coin is to use a boiled egg. Let it cool, place it in a bag, mash it together with the shell and put the fat-free item into the mixture. After half an hour, a “patina of antiquity” will appear on the jewelry, and 5-6 hours of exposure will give a durable iridescent patina.
Patination and oxidation
The surface of many metals (and copper is one of them), when interacting with the surrounding air and various chemicals, begins to become covered with a thin layer of oxides and oxides. This process, which also leads to a change in the color of the metal surface, is called oxidation. For the most part, the process of metal oxidation occurs naturally, but people have learned to cause it artificially, in industrial or home conditions, which is done to give the product an aged look.
Oxidation should not be confused with patination, a process whose essence lies in the fact that a thin layer of sulfur or chloride compounds is formed on the surface of the metal when interacting with various chemical elements. Patination, which, like oxidation, is accompanied by a change in the color of copper and bronze, can also be performed artificially using special compounds.
Copper aging occurs naturally over time or immediately when the surface is treated with any preparations.
If under natural conditions the process of oxidation and patination of copper or bronze can take years, then when using special solutions, patination occurs in a very short period of time. The surface of a product placed in such a solution literally changes its color before our eyes, acquiring a touch of noble antiquity. Using various chemical compositions, you can perform such procedures as blackening of copper, patination of objects made of copper and bronze, and blackening of brass in production and even at home.
Composition with sulfur liver
Liver sulfur, or baked sulfur, is a sulfur compound of sodium or potassium obtained by fusing sulfur powder with potash (wood ash) or soda. It is sold in chemical stores in the form of granules, powder or gel. You can prepare the composition with soda yourself:
- melt 20 g of sulfur and add soda to it until the mixture stops bubbling;
- add 50 ml of water, stir and boil for 2 minutes.
When the liquid has settled and cooled, it is drained, leaving a sediment, and stored in the refrigerator in a sealed opaque jar for 2-3 days. To make a solution from purchased raw materials, take 10-20 g of powder or granules per liter of water, and the gel is applied unchanged or diluted. In a heated liquid, the reaction is more active, but you can warm up the part itself under running hot water and lower it into a cold solution. The color of the film varies from reddish brown, purple, deep purple to black and depends on the concentration, temperature and exposure time.
The composition for blackening copper can ruin the inserts in a ring, pendant, or earrings. Pearls and some stones - turquoise, agate, jasper - are susceptible to reagents.
Methods for patination of copper at home
Applying an artificial patina is similar to the natural oxidation of a copper surface. But some methods are not durable and require fixing the film with varnish. Of the most common technologies, the chemical method, electrochemical and thermal are successful. Each of them requires certain compounds and tools, as well as compliance with safety measures.
Preparatory activities
Some methods of patination of copper require the use of toxic chemical compounds that emit toxic fumes. This requires compliance with certain rules during operation:
- the procedure for applying the oxide film is carried out in special cabinets or in a well-ventilated room;
- chemicals are kept in sealed containers to prevent spillage and the formation of harmful fumes;
- Before patination, the copper product must be thoroughly cleaned, degreased with alcohol and washed in well-warm water.
Before patination, the copper product must be degreased with alcohol.
After the patination procedure, the treated items are washed and laid out to dry. It is best to use sawdust for this purpose. They effectively cope with their task without damaging the oxide film, which has not yet been fixed with varnish. In addition, the fabric is not able to completely remove moisture from the relief surface and the patination process in hard-to-reach places will continue. The use of sawdust eliminates this, because... they completely draw out moisture.
Patination methods and their features
The first chemical method involves the use of ammonia. This is a rather aggressive substance, so the procedure must be carried out outdoors or in a well-ventilated area. In addition, you need to take care to protect your hands and face.
For patination you will need the following tools and materials:
- unnecessary plastic container with a lid;
- paper towels;
- ammonia solution;
- salt.
Line the bottom of the container with crumpled paper towels. An ammonia solution is poured onto them so that it completely saturates them. Salt is poured there, evenly distributing it around the perimeter. Copper objects are placed on the resulting pillow, slightly pressing them against the towels so that the bottom and sides are immersed in ammonia and salt.
The top of the product is sprinkled with salt over all surfaces and covered with another crumpled towel soaked in ammonia. There should be enough paper so that it can completely cover the copper. After this, the container is closed with a lid and left for several hours or days, depending on what result is desired.
From time to time you need to check the quality of patination so as not to overexpose the product and not get a darker color than you would like. A thin layer of patina will begin to form within a few minutes, but it will take at least a couple of days to achieve the antique look.
After the desired layer of patina has formed on the copper surface, the product is removed from the container and laid out on a clean piece of cloth or towel to dry. When the copper is dry, the remaining chemical solution is washed off and dried again. If the patina layer turns out darker than expected, you can lighten it with steel wool.
When the oxide film adheres well enough to the copper surface, it is coated with colorless varnish or paraffin to preserve the resulting patina.
The technology of patination of copper with baked sulfur has some rules. The composition cannot be used for blackening jewelry inlaid with stones. Semi-precious stones (jasper, agate, turquoise) and pearls are susceptible to chemical reagents. They either need to be pulled out before coating or another way to process the products must be found.
Liver of sulfur is a mixture of sodium or potassium, which is obtained by fusing sulfur powder with wood ash. The reagent is sold in any store in the form of granules, powder or gel. If you take soda instead of ash, you can make the composition yourself. To do this, melt 30 g of sulfur, while the mixture bubbles, add soda to it. 50 ml of water is poured there, stirred and boiled for 2-3 minutes.
The cooled mixture is filtered, leaving a sediment. It is stored in a tightly closed glass container for no more than 3 days. If a purchased reagent is used, it is prepared according to the instructions indicated.
It is advisable to warm up the liquid sulfur liver a little so that the patination process goes faster. The copper part is lowered into the solution and left for the required amount of time. The final color of the film depends on the concentration, temperature and exposure time. At the end of the procedure, the product is removed from the chemical, washed and dried.
Ammonia solution - a means for patination of copper
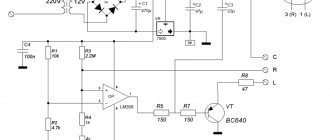
To give copper items a decorative appearance, electrochemical patination is used. The shades of the oxide layer depend on the temperature of the solution, the applied voltage, the exposure time, the texture of the products and the time they are kept in the electrolyte.
The electrolyte solution is prepared from the following components:
- 60 g of copper sulfate;
- 90 g sugar;
- 45 g caustic soda;
- distilled water.
300 ml of water is heated to 60-80 degrees. Copper sulfate and sugar are dissolved in it. Dissolve soda in another 300 ml, pouring it into the liquid gradually. Add the first composition to the second solution in small portions and bring the volume to one liter. The result should be an opaque dark blue liquid.
The electrolyte solution is heated, a copper piece is dipped into it and held for 1-2 minutes. After this, I increase the voltage based on the size of the product: from 0.6 to 1.2 V. For example, at 0.8-1 V an oxide layer of golden-pink patina is obtained, and at 1.3-1.5 V a film is formed fuchsia color turning into green. Further aging in the electrolyte suggests blue and purple hues.
There is a table by which you can track the dependence of the color of the patina on the time of exposure to the object using the galvanic method.
Interestingly, if you sharply increase the voltage in the electrolyte to 2 V for literally two seconds, you can get an oxide film with a chameleon effect. That is, the decoration will shimmer in different shades, depending on what angle you look at it from.
Caustic soda is needed to prepare an electrolyte solution
Patination of copper at home using an electrochemical method forms a film that is safe for human skin and quite durable. But to increase its service life, it is better to treat the surface with acrylic varnish, for example, “Plastik 71”. It will protect the copper jewelry from moisture and damage. Unlike many other compositions, this varnish erases and does not change the color of the patina on the metal.
Applying an oxide film using copper sulfide gives the alloy the desired shade, which can be varied by the saturation of the sulfur solution. The black chemical is produced by the reaction of divalent copper salts with sulfur. At the same time, it does not dissolve in water and dilute acid compositions, so the resulting patina will be quite stable.
If the resulting color deposit is too dark, it can be lightened by rubbing with cleaning powder. Then the copper object is washed with water and dried.
There are a couple more ways to patina copper that do not involve the use of chemicals. The simplest of them is heat treatment. Copper reacts instantly to high temperatures. The longer the firing takes place, the darker the color of the oxide film becomes. After the procedure, the material is immersed in water to consolidate the effect and coated with varnish.
The second way to add antique chic to brass is by using eggs. They are boiled, cooled, sliced or mashed with a fork. The crushed product is placed in an airtight container, and decorations are also placed there. The sulfur released by boiled eggs will affect the copper and within half an hour the first result will appear. The longer the exposure time, the stronger the effect.
Oxidation in ammonia vapor
Ammonia, or a 10% aqueous solution of ammonia, is used to tint copper in brown, olive color (in the recesses - to blue and black), spotted blue interspersed with copper. If you immerse the part in a solution of table salt before processing, bright blue or dark blue inclusions will appear on the dark surface.
The smooth surface is pre-sanded with sandpaper or a sander with attachments: then the film will lie more evenly and its tone will be more uniform. The textured surface is not sanded - differences in tone in the recesses and roughness create an additional artistic effect.
Each of the options for patination of copper in ammonia vapor has its own disadvantages and advantages; this does not affect the color of the film. Keep in mind that ammonia can damage tinted agates, malachite, natural pearls, and turquoise. It is safe for quartz (amethysts, citrines), garnets, glass, and Swarovski stones.
1) The solution is poured into a glass (one cap of a plastic bottle), placed on the bottom of the container, objects to be processed are laid out around and the lid is closed. Copper darkens after 30-60 minutes and acquires a dark olive color. This method is convenient for oxidizing a large number of parts, but due to the low concentration of ammonia in the air, the process can last from 4 to 6-8 hours for other metals.
2) The part is immersed in the solution. And if you need to age parts of the jewelry, the inserts of which are not resistant to ammonia solution, they are suspended in a glass container, onto the bottom of which ammonia is poured. To do this, use a wire with a cross-section of 0.4 or 0.3 mm: its free end is brought out through the holes in the lid. With this method, oxidation occurs in 10-15 minutes.
If it is not possible to hang an object, small parts are placed on stands made of caps, and large parts are fixed on a frame made of wooden sticks. With this processing method, fewer items are painted at the same time.
Once you have the desired shade, remove and rinse the part. Sometimes the process needs to be repeated as the final patina color develops as the copper dries. The finished decoration is coated with wax or varnish in an aerosol, for example, Krylon Matte Finish.
Patination in sawdust
For work, clean wood waste is used. Sawdust from laminated plywood cannot be used, as the glue reacts with the patination solution. In this way, speckled pink, blue and black shades are obtained on copper, and speckled gold, green or blue, brown, black are obtained on brass. The solution is prepared from:
- 16 grams of salt;
- 300 ml (for brass and other alloys) / 600 ml (for copper) ammonia;
- 700 ml water.
Mix the ingredients and soak the sawdust in the solution - they should be damp, but not wet. Place the mixture in a plastic container, immerse the product in it, and seal the container. Place a couple of pieces of copper close to the surface of the mixture to control the coloring by their color without touching the workpiece.
The solution can be used not only with sawdust, but also with other moisture-absorbing carriers: dry leaves, hay, cat litter. The processing time depends on the medium in which the metal is painted (for copper in sawdust it is 4-6 hours, for brass - from 12 hours to a day) and on the structure of the medium. Fine-textured materials should not be stirred during the process, and the product in leaves or hay must be rotated so that the coloring occurs evenly.
Also, the color intensity and other parameters of the oxide film are affected by:
- Proportions of ammonia and/or salt.
- Carrier moisture content. If the sawdust is not wet enough, the coloring will be weak; if it is wet, a mottled color will not work (the surface of the copper will be etched).
Wash and dry the parts in dry sawdust. The finished mixture is suitable for several days, and the solution in an airtight container in the refrigerator or cellar is stored for quite a long time.