Cast aluminum alloys
The main alloying elements of cast aluminum alloys are magnesium, copper and silicon. They provide a qualitative change in the nature of aluminum alloys. Intermetallic compounds are formed in Al-Cu, Al-Mg and Al-Mg-Si alloys, and eutectic is formed in Al-Si alloys. Intermetallic compounds, especially in combination with eutectics, make it possible to use various methods of thermal hardening. Other alloying elements - auxiliary and modifying - are used in much smaller quantities to improve the specified mechanical and physical properties of alloys.
Alloys based on aluminum and magnesium. Properties and applications
Aluminum is well processed by pressure, easily welded in argon, but difficult to cut. In air it quickly oxidizes and becomes covered with a thin dense film of oxide, which does not allow oxygen to penetrate into the thickness of the metal, which provides protection against corrosion. Aluminum in its pure form is practically never used. To increase the strength characteristics, aluminum is alloyed with various metals and non-metals (copper, magnesium, silicon, iron, titanium, etc.); wrought aluminum alloys are used to produce sheets, strips, wires, shaped profiles, using stamping, pressing, and forging methods.
Aluminum alloys are divided into two groups according to the method of manufacturing products from them: wrought and cast. This division reflects the basic technological properties of alloys: wrought alloys have high ductility when heated, and cast alloys have good fluidity. To obtain these properties, different alloying elements are introduced into aluminum and in unequal quantities.
Among non-hardening aluminum alloys, alloys based on AI-Mn and AI-Mg have acquired the greatest importance. Manganese and magnesium, like copper, have limited solubility in aluminum, which decreases with decreasing temperature.
Applications Most aluminum alloys have high corrosion resistance in the natural atmosphere, sea water, solutions of many salts and chemicals, and in most food products. The latter property, combined with the fact that aluminum does not destroy vitamins, allows it to be widely used in the production of tableware. Aluminum alloy structures are often used in seawater. Aluminum is used in large quantities in construction in the form of cladding panels, doors, window frames, and electrical cables.
Aluminum alloys are not subject to severe corrosion over a long period of time when in contact with concrete, mortar, or plaster, especially if the structures are not frequently wet. Aluminum is also widely used in mechanical engineering, because... has good physical qualities.
Magnesium. The addition of magnesium significantly increases strength without reducing ductility, increases weldability and
increases the corrosion resistance of the alloy. Magnalium is harder and stronger than pure aluminum, the latter is easier to process and polish
Magnesium alloys. Magnesium alloys are light, characterized by high specific strength, as well as good casting properties and are excellent in cutting. Therefore, they are used to make parts for rockets and aircraft engines, car body housings, wheels, gas tanks, portable tables, etc. Some magnesium alloys, which have a high viscous damping coefficient, are used for the manufacture of moving machine parts and structural elements operating under conditions of unwanted vibrations.
Magnesium alloys are quite soft, have poor wear resistance and are not very ductile. They are easily formed at elevated temperatures, are suitable for arc, gas and resistance welding, and can also be joined by soldering (solder), bolts, rivets and adhesives. Such alloys are not particularly corrosion resistant to most acids, fresh and salt water, but are stable in air and are usually protected from corrosion by surface coating - chrome etching, dichromate treatment, anodizing. Anodizing magnesium alloys increases their surface hardness and abrasion resistance.
Magnesium oxide is used in the production of cements, refractory bricks, and in the rubber industry. Magnesium peroxide (“Novozon”) is used to bleach fabrics
Magnesium oxide and salts are used in medicine (asparkam, magnesium sulfate, magnesium citrate, bischofite mineral). For the treatment of the musculoskeletal system, nervous and cardiovascular systems
Petrol. Requirements, properties, labeling.
Gasoline is a complex mixture of light aromatic, naphthenic, paraffinic hydrocarbons and their derivatives with a number of carbon atoms from 4 to 10 and an average molecular weight of about 100.
· has high carburetion properties i.e. forms a combustible mixture that ensures easy engine starting and stable operation) in all possible modes
· does not cause engine detonation i.e. has sufficient detonation resistance
Ensures complete combustion and does not cause tar or carbon deposits in the engine
· has high stability i.e. during long-term storage, pumping and transportation, the composition and properties of gasoline remain without significant changes
· - during storage does not cause corrosion of the metal in the tank tank, and during combustion of engine parts from the action of combustion products
· the heat of the combustion products of the combustible mixture should be max.
Volatility. The volatility of gasoline determines the possibility and speed of starting the engine, the reliability of its operation in given climatic conditions, the consumption and loss of gasoline during transportation, storage and pumping. Volatility is a performance property that is no less important for gasoline than its anti-knock characteristic, which is strictly regulated by standards.
Oxidability. The tendency of motor gasoline to oxidize is assessed by such indicators as acidity, actual resins, and induction period. Its oxidability depends on the access to atmospheric oxygen, temperature, the chemical composition of gasoline and contact with metals that catalyze its oxidation. The oxidability of gasoline components varies greatly. Heteroatomic compounds are most susceptible to oxidation, followed by unsaturated hydrocarbons. During long-term storage of motor gasoline, the tetraethyl lead contained in it also undergoes oxidative decomposition with the formation of an insoluble phase. Motor gasolines are stabilized with antioxidant additives (oxidation inhibitors).
Aluminum-magnesium alloys
Aluminum-magnesium alloys are single-phase binary alloys with medium to high strength levels and good toughness properties. The fact that they are single-phase means that they are not able to increase their strength as a result of heat treatment.
The main feature of these Al-Mg alloys is their high corrosion resistance, including in sea water and the marine atmosphere. The highest corrosion resistance is achieved with a minimum of impurities - both solid and gaseous. Therefore, these alloys are made from high-quality metals and with special care when melting and casting them. These alloys are easy to weld and are often used in construction for decorative finishing. Aluminum-magnesium alloys are easy to cut and have an attractive appearance after anodizing.
Magnesium-aluminum system
Due to the fact that magnesium-aluminum alloys form the basis of a significant number of high-strength technically important magnesium alloys, the Mg-Al system has been studied intensively.
The main factors determining the formation of these alloys have already been discussed earlier; Here we only recall that the volume factor of aluminum relative to magnesium is on the border of the favorable zone. This is what causes a sharper decrease in the solidus curve in the magnesium-rich region of the equilibrium diagram compared to the corresponding solidus curves for Mg-In and Mg-Tl alloys. Hence the lower solubility limit of aluminum in magnesium. A sharp decrease in the solubility of aluminum in magnesium in the solid state with decreasing temperature also indicates a significant difference in the atomic sizes of these elements. All of the above is also true for alloys of this system rich in aluminum. In Fig. 161 shows an equilibrium diagram of the Mg-Al system, constructed based on data from a large number of experimental works. The liquidus curve, which can be considered established quite accurately, was constructed based on the data from the works listed in the link. On the magnesium side, the curve smoothly decreases down to the eutectic at 32.3% wt. (30.07% atomic) Al, while on the aluminum side the liquidus curve smoothly decreases to the eutectic at 65 wt%. (62.6% atomic) Al. The data of various researchers in the part of the liquidus curve between these two eutectic points differ from each other. In Fig. 161 shows the most probable liquidus curve, constructed according to recently published work, which will be discussed in detail below. According to these data, the liquidus curve from the eutectic point at 437°C rises to 462°C at the point corresponding to 45.4-46% wt. (42.84-43.43% atomic) Al; at this point the liquidus and solidus curves touch. Next, the liquidus curve slowly decreases to the eutectic point at 450.5 ° C, corresponding to 60.7% wt. (68.20% atomic) Al. As the aluminum content increases further, the curve passes through a small maximum before reaching the aluminum-rich eutectic at 460°C. It is not surprising, therefore, that different results have been obtained by different researchers in this area.
Solidus curves are also known quite accurately; in particular, the data on the solidus curve on the magnesium side, obtained by researchers using various methods, coincide well. The curve intersects the eutectic horizontal (437° C) at 12.6% wt. (11.50% atomic) Al.
A critical examination of the work indicates that the solubility of aluminum in magnesium in the solid state gradually decreases with decreasing temperature, reaching 2.1 wt.% at room temperature. (1.90% atomic) Al as shown in Fig. 161. This curve is established with an accuracy of ±0.2 to ±0.3; the solidus curve on the aluminum side and the solubility curve of magnesium in aluminum in the solid state have also been established quite accurately.
Subsequently, Hansen and Geiler confirmed the existence of this intermediate phase with a maximum on the liquidus curve; the maximum position, however, is only approximately established in the region from 45 to 50% wt. Al, since it is not clear whether it exactly matches the composition of Mg4Al3. The authors concluded that the solidification range for this composition is approximately 5° C. According to their data, the liquidus and solidus curves touch at the point corresponding to the Mg3Al2 composition. Since, however, the solidification point for this composition is lower than for the Mg4Al3 composition, and since the liquidus curve decreases towards the magnesium-rich eutectic, this conclusion appears to be incorrect from a thermodynamic point of view.
Hansen and Geiler also showed that the system has a second intermediate phase with a narrow homogeneity region on both sides of the Mg2Al3 composition, which also corresponds to a weak maximum on the liquidus curve. Despite the difficulties of metallographic analysis, the authors showed that at temperatures close to the melting point, this intermediate phase occupies a region from 40 to approximately 57 wt.%. (37.54 - 55.44% atomic) Al. The phase rich in aluminum and having a narrow homogeneity region was designated by p, and the phase of the expected composition Mg4Al3 or Mg3Al2 was designated by y. These notations were also used in constructing the equilibrium diagram shown in Fig. 161.
Subsequent studies showed that in reality the Mg-Al system is more complex. Thus, Kawakami, based on a metallographic study of alloys containing from 42 to 62% wt. (39.50-63.63% atomic) Al, showed that in the Mg-Al system there is also a third intermediate phase (Fig. 162), which he designated by K. The K phase is formed at 455 ° C along the periphery tectic reaction between liquid and y-phase; it reacts peritectically with the liquid, forming the b-phase at 450°C. According to Kawakami, the K-phase has a relatively noticeable region of homogeneity only above 430 ° C; below this temperature it exists in the region of 54.2-54.6 wt% (51.61-52.01 atomic%) Al. The phase boundary on the aluminum side at temperatures above 430 C almost exactly coincides with the y/(y + b) boundary proposed by Hansen and Geiler. Although Kawakami established the presence of the K phase based on metallographic and X-ray analysis, it should be noted that no other researcher has discovered the peritectic reaction associated with the formation of this phase.
Köster and Dullenkorff suggested that since the K phase was found after annealing the alloys, it is possible that the diagram proposed by Kawakami corresponds to a stable equilibrium, while the diagram containing no K phase corresponds to a metastable state of the alloys. This assumption was confirmed by the results of a study of two alloys of the corresponding composition, slowly cooled from the melt, but containing small additions of zinc. In one case (53.5% Al, 43.5% Mg, 3% Zn) the primary crystals of the y-phase were found to be surrounded by a shell of what appeared to be the K-phase, which was surrounded by the beta-phase. In an alloy containing 57.5% Al, 37.6% Mg and 5% Zn, only y- and beta-phases were found.
Subsequently, Riederer, who studied annealed and quenched alloys, previously quickly cooled from the liquid state, did not discover the K phase in them. The author confirmed the correctness of the equilibrium diagram of the Mg-Al system constructed by Hansen and Geiler and containing the b- and y-phases. However, after very slowly cooling an alloy containing approximately 55% Al and a small amount of zinc, Riederer metallographically detected a phase different from the y phase. Subsequently, he discovered the same phase in an alloy that did not contain zinc. During subsequent annealing, grains of the new phase, which was classified by the author as the K phase, grew at the expense of the y phase. Since the K phase was not observed in samples annealed after quenching and since it remained stable once it appeared, Riederer concluded that this phase could only form directly from the melt. He also x-ray studied all the intermediate phases and showed that the p-phase has a hexagonal structure (later Perlita refuted this conclusion), the y-phase is isomorphic with a-Mn and contains 68 atoms in a unit cell, and the K-phase is characterized by a complex diffraction a picture that cannot be deciphered.
Subsequently, Lavey and Muller came to the conclusion that there is a fourth intermediate phase in the Mg-Al system. Alloys containing from 47.6 to 52.6% wt. (45.02-50.01% atomic) Al and quenched from the liquid state contain a y-phase, which was established both metallographically and x-ray (a-Mn type). In an alloy containing 53.6% wt. (51.0% atomic) Al, a small amount of another phase was detected metallographically, which can be called the y' phase. It was etched in almost the same way as the y-phase, but turned out to be very anisotropic in polarized light. With increasing aluminum content, the proportion of this phase in the alloys increased in the range from 56.6 to 57.5 wt%. (54.03-54.94% atomic) Al, it was observed in its pure form. At 60% wt. (57.48 atomic%) Al traces of the b-phase appeared.
Based on the analogy of the compositions, we can conclude that the y'-phase of Laves and Müller should be identified with the K-phase of Kawakami. However, after three days of annealing at 300°C, a new phenomenon was discovered. Melts containing from 61.6 to 52.6% wt. (49.0-50.01% atomic) Al, which, according to Kawakami, should consist of y- and X-phases at 300°C, contained only y- and p-phases. Similarly, in alloys that, after quenching from a liquid state, were supposed to contain a homogeneous K(y') phase, this phase was not detected after annealing at 300° (C). However, the β and y phases were observed in approximately equal quantities, and also a small amount of a third metallographically distinguishable phase, which can be designated by c'.
Thus, it is clear that the K or y' phase is not stable at 300° C. The nature of the beta phase, however, is not clear. All alloys containing from 64.6 to 60% wt. (52.02-57.48% atomic) Al and annealed at 300° C, contained three phases b, y and b'. In order to test the assumption that the β'-phase may represent a transition phase formed during the decomposition of K(y') into a and y, and should disappear upon further heating, long-term annealing was carried out at 300°C for nine days. As a result of such heat treatment, the amount of β'-phase in the alloy increases. Laves and Müller did not obtain this phase in pure form, but since at 58.5% wt. (55.96% atomic) Al only a small amount of the y-phase was detected, the authors suggested that the beta-phase has a narrow homogeneity region around 595 wt%. (56.92% atomic) AL Subsequently, it was shown that the b'-phase disappears as a result of annealing the alloy at 300° C and then at 400° C.
Consequently, at a certain temperature in the region from 300 to 400° C, the β' - b + y transition occurs, while at a certain temperature between the solidus line and 300° C, the K (y') phase becomes unstable relative to b'.
Although this part of the equilibrium diagram cannot be considered definitively established, Laves and Müller indicated that the most likely type of equilibrium diagram for the Mg-Al system is that corresponding to that shown in Fig. 163. They found that the details of the observed effects depended largely on the impurities and, in particular, found that the b' phase was stabilized to some extent in the presence of a small amount of zinc. The authors showed that the y'-phase has a crystal lattice of deformed a-Mn and, therefore, the structures of the y- and y'-phases are very close. The x-ray diffraction pattern of the b'-phase turned out to be identical to the x-ray diffraction pattern of Riederer's K-phase. Thus, it is clear that Riederer did not obtain the same phase as Kawakami, but discovered for the first time the β'-phase, without, however, specifying the temperature range of existence of this phase, which is between 300 and 400 ° C.
From what has been considered it follows that the central part of the equilibrium diagram of the Mg–Al system is quite complex. The work of Kurnakov and Mikheeva brought significant clarity to this issue. In three papers devoted to this system, the authors showed that certain temperature stops can be observed when heating alloys containing from 49 to 59% wt. (46.41-56.47% atomic) Al, and that these stops vary with composition in the manner shown by the dotted curve in Fig. 161. This thermal effect signifies a transformation in the solid state, and the smooth change in temperature with change in composition is consistent with the existence of a two-phase field in this composition region between the y-phase and the putative K-phase.
Thus, the most probable type of equilibrium diagram includes the y-phase, which reaches 59% wt. (56.47% atomic) Al at the liquid-b+y-eutectic transition temperature (480.6°C). This value is close to the boundary of existence of the K-phase on the aluminum side, the crystal structure of which is a distorted structure of the y-phase.
It is most likely that in the considered middle part of the equilibrium diagram at high temperatures, only the b- and y-phases exist. The y-phase has a slightly larger region of homogeneity than expected and transforms into a disordered form near the boundary of the homogeneity region on the aluminum side. This transition, according to the data of Kurnakov and Mikheeva, is associated with a noticeable change in the direction of the y/(y + b) phase boundary. This is also evidenced by the thermal stop in the region (b+y), discovered but not considered by Hansen and Geiler. It should also be noted that the maximum transformation temperature in the solid state for the y-phase lies near the AlMg composition.
The presence of the β-phase in alloys slowly cooled or quenched at temperatures of 370-390 ° C was confirmed by Kournikov and Mikheeva. The authors showed that when zinc is introduced into an alloy, the β'-phase can turn out to be quite stable. In this case, it crystallizes directly from the melt. Hence, it was suggested that in binary alloys this phase is a metastable component. Metallographic studies, however, indicate that the β'-phase can also be a stable component of a binary system (due to the lack of clarity of this issue, it is shown as a dotted line in the equilibrium diagram in Fig. 161).
In conclusion, it can be noted that the equilibrium diagram of the Mg-Al system shown in Fig. 161 seems to best “reflect the somewhat contradictory experimental data obtained by many researchers. It is most likely that the Kawakami K phase is absent in the system; The metallographic data obtained by Laves and Müller for the highest temperature phase are most likely due to etching effects associated with transformation within the y phase.
The lattice parameters of the primary solid solution of aluminum in magnesium were discussed above. It is interesting to consider the results of X-ray studies of intermediate phases. Although, according to the studies, the b-phase has a hexagonal structure, isomorphic with Cu4Cd3, it is more likely that it has a very complex structure. The crystal structure of the (C-phase) is unknown; it is only clear that the X-ray diffraction pattern of this phase is complex.
As already indicated, the y-phase has a crystal structure of the a-Mn type. According to Ridarer, who confirmed the data of Laveca et al., the lattice constant of the y-phase varies linearly from 10.45 A at 52.7% Al to 10.56 A at 40.5% Al. From Fig. 161 it can be seen that these period values correspond to the phase boundaries on the aluminum and magnesium side, respectively.
Delinger, based on the similarity of the structures of the y-phase and a-Mn, came to the conclusion that this phase is most likely based on a compound whose ideal composition is Mg17Al12; elementary; the cell in this case contains two “molecules”.
Pelzel measured the densities of Mg-Al alloys in solid and liquid states in order to determine the compression during solidification and the change in specific volume during the formation of the alloy from the original elements. The author found that in Mg-Al alloys the reduction in volume upon solidification, amounting to 3.97% for pure magnesium, increases with increasing aluminum content up to 32% Al. Further, the compression decreases slightly near the composition Mg17Al12 and increases again with further addition of aluminum. The obtained values are given in table. 21.
More important data are the compression observed during the formation of alloys, i.e., the difference between the observed specific volumes and those calculated by the mixing rule. In table Figure 21 shows these compression values, as well as linear expansion coefficients obtained from measurements in the solid state.
It is interesting to note that in both solid and liquid states the greatest compression is observed in the composition range corresponding to the two main intermetallic compounds. ) In the Mg17Al12 compound, the heteropolar nature of the bonds is most likely and, apparently, a similar phenomenon occurs in the Mg2Al3 compound. From this point of view (i.e., based on the interaction between the atoms of the components), the observed decrease in specific volumes becomes clear. It is also interesting to note that the nature of the change in compression with composition is almost similar to the nature of the change in the heat of mixing or formation of alloys, so that the factors that cause compression are also responsible for thermochemical effects.
A significant decrease in the specific volume in the liquid state indicates the presence of interaction between the atoms of the components not only in the solid, but also in the liquid state.
Alloys difficult to cast
Compared to aluminum-silicon alloys, all aluminum-magnesium alloys have significantly more problems during casting. They require more careful mold design and higher solidification temperature gradients to produce good castings.
When casting these alloys, one must take into account their increased tendency to oxidize during melting. This is also important because many products made from these alloys require high surface quality and oxide defects are highly undesirable.
Areas of application of aluminum alloys
Areas of application of aluminum and its alloys:
- Cutlery. Aluminum utensils, forks, spoons and containers for storing liquids are still popular.
- Food industry. This metal is used as a food additive. Its designation in the composition of products is E. It is a food additive used to color confectionery products or protect products from mold.
- Rocket science. Aluminum is used to make rocket propellant.
- Military industry. An acceptable price and low specific gravity have made this metal popular in the production of parts for small arms.
- Glassmaking. This material is used in the manufacture of mirrors. This is due to its high reflectivity.
- Jewelry. Aluminum jewelry used to be very popular. However, it was gradually replaced by silver and gold.
Due to its high electrical conductivity, this metal is used for the manufacture of wires and radio components. In terms of electrical conductivity, aluminum is second only to copper and silver.
We must not forget about the small specific gravity of the material. Aluminum is considered one of the lightest types of metal. Due to this, it is used to make housings for aircraft and cars. Delving deeper into this topic, we can say that the entire aircraft consists of at least 50% of this metal.
This metal is also found in the human body.
If this component is missing, the processes of tissue growth and regeneration slow down. The person feels tired, muscle pain and increased drowsiness may appear. However, more often situations arise when this component is more than normal in the body. Because of this, a person becomes irritable and nervous. In case of excess, it is necessary to abandon cosmetics with the addition of aluminum and medications containing it. Mixtures with aluminum are common in various industries. This is due to the fact that this metal is among the top 5 most common in the world. In nature it is found in various ores. In production, the weak performance of this metal is increased by adding other components. This way you can increase resistance to corrosion processes, strength, and melting point.
Cast aluminum alloy 514.0
Alloy formula: 4Mg
Chemical composition:
- copper: 0.15% max;
- magnesium: 3.5-4.5%;
- manganese: 0.35% max;
- silicon: 0.35% max;
- iron: 0.50% max.
- zinc: 0.15% max;
- titanium: 0.25% max;
- others: each 0.05%, sum 0.15% max.;
- aluminum: the rest.
Typical mechanical properties (as delivered):
- tensile strength: 145 MPa;
- yield strength: 95 MPa;
- relative elongation: 3%;
- Poisson's ratio: 0.33;
- modulus of elasticity: 71.0 GPa.
Physical properties:
- density: 2.65 g/cm3;
- liquidus temperature: 630 ºС;
- solidus temperature: 585 ºС.
Technological properties:
- melting point: from 675 to 815 ºС;
- casting temperature: from 675 to 790 ºС;
- welding alloy – 4043.
Cast aluminum alloy 518.0
Alloy formula: 8Mg
Chemical composition:
- copper: 0.25% max;
- magnesium: 7.5-8.5%;
- manganese: 0.35% max;
- silicon: 0.35% max;
- iron: 1.8% max;
- Nickel: 0.15% max;
- zinc: 0.15% max;
- Tin: 0.15% max;
- others: amount 0.25% max.;
- aluminum: the rest.
Typical mechanical properties (as delivered):
- tensile strength: 310 MPa;
- yield strength: 190 MPa;
- relative elongation: 5-8%.
Physical properties:
- density: 2.57 g/cm3;
- liquidus temperature: 620 ºС;
- solidus temperature: 535 ºС.
Cast aluminum alloy 520.0
Alloy formula: 10Mg
Chemical composition:
- copper: 0.25% max;
- magnesium: 9.5-10.6%;
- manganese: 0.15% max;
- silicon: 0.25% max;
- iron: 0.30% max;
- zinc: 0.15% max;
- titanium: 0.25% max;
- others: each 0.05%, sum 0.15% max.;
- aluminum: the rest.
Typical mechanical properties (as delivered):
- tensile strength: 330 MPa;
- yield strength: 180 MPa;
- relative elongation: 16%.
Physical properties:
- density: 2.57 g/cm3;
- liquidus temperature: 605 ºС;
- solidus temperature: 450 ºС.
Magnesium ingots for alloying aluminum alloys
Magnesium is the brother of aluminum
Magnesium is similar to aluminum in many ways. The density of magnesium at 20 °C is 1.74 g/cm³ - it floats in liquid aluminum (density of liquid aluminum is 2.4 g/cm³). The melting points of aluminum and magnesium are almost the same: for magnesium - 650 °C, for aluminum 99.5% - 657 °C. Therefore, magnesium is directly loaded into the melting furnace, unlike, for example, silicon. Pure silicon has a high melting point, 1415 °C. For this reason, silicon is introduced into the aluminum melt, usually in the composition of silumin with a silicon content of about 12%. This eutectic aluminum alloy Al-Si melts at a temperature of only about 577 °C.
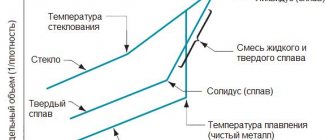
Aluminum-magnesium phase diagram
Source: Aluminum and Aluminum Alloys, ASM International, 1996
Are there metals lighter than aluminum?
The development of the production of polymer and composite materials does not at all relegate light metals to the background. Their production volume continues to grow, especially for aluminum and its alloys. But there are also “more other” light metals and alloys.
The lightest
- all alkali metals: lithium, sodium, potassium, rubidium, cesium and francium. But in industry, only lithium is used as a light material, and even then in alloys.
Lithium
- the lightest of metals, it is almost twice as light as water. But, unfortunately, it is impossible to use it in its pure form as a structural material - it is too active and interacts with both water and air. But in alloys, for example, with aluminum - please.
Modern aluminum-lithium alloys are superior to traditional alloys in many respects, and in some cases compete with fiber-reinforced plastics. The required viscosity of such alloys is ensured by the introduction of alloying elements into them - manganese, zirconium and copper, appropriate material manufacturing technology, leading to the formation of a fine-grained structure, as well as the use of thermomechanical treatment, which affects the uniformity of the separation of dispersed phases.
Thanks to this, the required combination of strength and toughness is achieved, which, with low density and high rigidity, makes it possible to consider aluminum-lithium alloys as the most promising material for lightweight structures.
Aluminum-lithium alloys are interesting for the aerospace industry. This is because the addition of lithium simultaneously increases the elastic modulus and reduces the density of the alloy, thereby helping to reduce the weight of structures.
Of the alkaline earth metals, only magnesium and beryllium are used in structural materials. Calcium and strontium, despite the fact that they are lighter than aluminum, are practically not used.
Magnesium alloys are numerous and are divided into two groups: casting and wrought.
Due to their low density and high specific strength, they are used in aerospace engineering as structural materials. Magnesium makes them light - magnesium alloys are a third lighter than aluminum.
About a fifth of the smelted magnesium is used in the automotive industry.
Magnesium
It is also preferable in the manufacture of composites with a metal matrix. Molten aluminum, for example, reacts with silicon carbide reinforcing fibers to form aluminum carbide. Magnesium is inert towards these fibers.
Beryllium
simultaneously refers to both light and rare metals, and therefore also expensive. But the main disadvantage of this metal, which prevents its use in technology, is its high toxicity.
Another negative factor is the fragility of beryllium. However, it has much more advantages.
Beryllium is one of the lightest metals. It is 1.5 times lighter than aluminum and 4 times lighter than stainless steel. In terms of elasticity modulus, it surpasses steel, titanium, and aluminum. It is important that its strength properties are not lost up to a temperature of 800 degrees.
Despite its high cost and toxicity, beryllium is used where it is indispensable. Thus, in the USA, panels of the Agena series of spacecraft, parts of the antenna on the Texet-1 communications satellite, and the reflector of the Nerva-1 nuclear rocket engine were made from it.
Aluminum is in the third group of elements. There are no other metals lighter than aluminum in this group. In the fourth group, only silicon is lighter than aluminum. But its significance is so great that it deserves a separate article.
Let's summarize. There are many metals lighter than aluminum, more than a dozen. But not all of them, alas, can replace or supplement it.
However, everything is changing...
Tags: creative thought, benefit, physics, interesting fact, metal, chemistry