What it is?
The comparison of this metal with gold is based only on external similarity, since in terms of chemical composition, characteristics and other features, these two metals are completely different. Although brass has been known to mankind for a long time, they first started talking about tombac only in the eighteenth century - after the discovery made by Christopher Pinchbecker. At that time, a similar alloy was actively used in England. And only a few decades later its popularity spread to other countries.
On the similarity of tombac to a jewelry mineral, many dishonest people were able to make good money by passing off coins and jewelry made of brass alloy as real ones made of gold.
The metal in question can be called similore, princetal, chrysorine, chrysochalk, ororeid. When you hear these names, don’t be surprised - they’re all the same metal.
Tombak has many advantages:
- good resistance to corrosion damage;
- excellent wear resistance;
- good weldability with other metals;
- plasticity is sufficient for the use of deformation and engraving methods;
- possibility of enameling and gilding;
- immunity to magnets.
One type of metal, called polutompak . Its difference from tombak lies in the percentage of zinc, which can range from 10–20%. This feature explains the change in the basic properties of the metal:
- rich yellow color;
- decreased plasticity;
- increased refractoriness.
Such characteristics make it possible to use semi-tombak for the production of some technical parts. This type of metal is less common compared to tombac.
This is due to the presence of a large number of deficiencies due to the high zinc content in it.
What is tompak?
Tompak is one of the many varieties of brass, which combines zinc and copper in a certain proportion. The comparison of this metal with gold is based only on external similarity, since in terms of chemical composition, characteristics and other features, these two metals are completely different. Although brass has been known to mankind for a long time, they first started talking about tombac only in the eighteenth century - after the discovery made by Christopher Pinchbecker. At that time, a similar alloy was actively used in England. And only a few decades later its popularity spread to other countries.
On the similarity of tombac to a jewelry mineral, many dishonest people were able to make good money by passing off coins and jewelry made of brass alloy as real ones made of gold.
The metal in question can be called similore, princetal, chrysorine, chrysochalk, ororeid. When you hear these names, don’t be surprised - they’re all the same metal.
Tombak has many advantages:
- good resistance to corrosion damage;
- excellent wear resistance;
- good weldability with other metals;
- plasticity is sufficient for the use of deformation and engraving methods;
- possibility of enameling and gilding;
- immunity to magnets.
One type of metal, called semi-tompak, deserves special attention. Its difference from tombak lies in the percentage of zinc, which can range from 10–20%. This feature explains the change in the basic properties of the metal:
- rich yellow color;
- decreased plasticity;
- increased refractoriness.
Such characteristics make it possible to use semi-tombak for the production of some technical parts. This type of metal is less common compared to tombac. This is due to the presence of a large number of deficiencies due to the high zinc content in it.
A little history
This alloy has been known since the times of ancient South American pre-Columbian civilizations; brass jewelry, dishes, and tools were found during archaeological excavations. Instead of zinc, the production secret of which was lost in Europe in the 10th-11th centuries and rediscovered only a few centuries ago, the alloy was made at that time using rich zinc-containing ore - galmey (a mixture of zinc spar ZnCO3 and zinc silicate).
The tombac was reinvented in England by the London watchmaker Christopher Pinchbecker (1670 - 1732). It is he who is considered the author of the chemical composition of this alloy. Initially, the alloy was used to make chains and watch parts. But soon tombak became widespread not only in England, it became known all over the world. Many fortunes were made by various scammers based on the similarity of tombac to gold; they became rich by passing off coins and items made from it as gold.
Marking
Tombak, cast in bars or made in the form of rolled sheets, is marked with the designation “L90”, where “L” is brass, and the number “90” means the percentage of copper content. Impurities in such an alloy are less than 0.2%. “L96” means that the alloy is 95-97% copper, no more than 0.2% impurities, the rest is zinc. If the number on the marking is “85” or less, then it is not a tombac, but a semi-tompak. And this means that the amount of zinc in the alloy is more than 10%, its ratio can reach up to 22%. The amount of additional components can reach 0.3%. By increasing the volume of zinc, the basic properties of the alloy change:
- the color changes, it becomes more yellow;
- the degree of plasticity decreases;
- refractoriness increases.
This material is more often used for the manufacture of technical parts; bellows, flexible hoses, condensation tubes, and wire mesh used in filter-driers for refrigeration machines are made from it.
A little history
This alloy has been known since the times of ancient South American pre-Columbian civilizations; brass jewelry, dishes, and tools were found during archaeological excavations. Instead of zinc, the production secret of which was lost in Europe in the 10th-11th centuries and rediscovered only a few centuries ago, the alloy was made at that time using rich zinc-containing ore - galmey (a mixture of zinc spar ZnCO3 and zinc silicate).
The tombac was reinvented in England by the London watchmaker Christopher Pinchbecker (1670 - 1732). It is he who is considered the author of the chemical composition of this alloy. Initially, the alloy was used to make chains and watch parts. But soon tombak became widespread not only in England, it became known all over the world. Many fortunes were made by various scammers based on the similarity of tombac to gold; they became rich by passing off coins and items made from it as gold.
Tompak - outdated names
Red brass
(French tombac, from Malay tambaga - copper) - a type of brass with a copper content of 88-97% and zinc up to 10%. It has high ductility, anti-corrosion and anti-friction properties. Copper alloys with a zinc content of 10-20% are called semi-tombacs. Tombak was reinvented by London watchmaker Christopher Pinchbecker (circa 1670-1732), and was known in ancient times to the Peruvian Moche civilization. Sometimes names are used for the alloy: chrysochalk, similor, oreid, chrysorin, princemetal, etc.
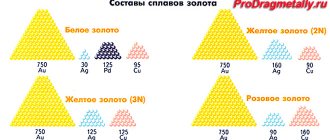
Composition of pseudo-precious brass
Brass has been known for a very long time, since the times of the ancient Romans, and this despite the fact that zinc was discovered only 5 centuries ago. In those distant times of antiquity, galmei (zinc ore) was added to the mentioned alloy instead of pure Zn. But the tombac that we know today was invented by Christopher Pinchbecker in the 17th century. The alloy became widespread not only in England; within a few decades people learned about it all over the world. And by the way, many scammers got rich by passing off coins, various jewelry and products made from this type of brass as gold. Today in everyday life you can find other names for this alloy - similor, princetal, chrysochalk, oreid and chrysorin.
As you may have guessed, it is an alloy of copper and zinc. The content of the first element ranges from 88 to 97%, and the second - no more than 10%. If you increase the share of Zn to 10–20%, then you get a semi-tompak. The content of the last element affects the properties of brass and its color. As it increases, the color changes from red to yellow. Sometimes tombac bars can be confused with precious gold in the photo. Such a change leads to improved antifriction, technological and mechanical properties, and the cost, on the contrary, decreases. This is due to the relative cheapness of zinc compared to copper.
Yellow brass
The material has excellent anti-corrosion properties and wear resistance. It welds perfectly with various steels and precious metals. It is distinguished by increased plasticity. Easily amenable to enameling and gilding, forging, drawing and engraving.
Another feature is the complete absence of magnetic properties.
Product made from tombak
The hardness of the material reaches 145 MPa. The temperature at which the melting process begins is 1045 °C. Without the use of lubricant, the friction coefficient is 0.44. If you use special lubricants, this figure can be reduced to 0.074. Tensile strength ranges from 440 to 520 MPa, and elongation after break is about 3%. Brass L90 has a density of 8780 kg/m3. To obtain the specified characteristics, additional elements are introduced into the composition of the tombac alloy. Their content is only a few tenths of a percent. The result is a material with an ideal ratio of ductility and strength, excellent appearance and reasonable price.
The alloy, depending on the further fate, can be sheet or in bars. The marking indicates the letter “L” first, followed by the percentage of copper. For example, L96 consists of 95–97% Cu, no more than 0.2% impurities and the rest is Zn. But if you see the designation L90 on a product, it means that the main element is contained within 88–91%, impurities are less than 0.2%. Brands L85 and L80 belong to semi-tompak. The share of additional components in them can reach 0.3%.
Chemical composition and main characteristics
An alloy of copper and zinc, which is well known to everyone under the name “brass,” has been used by people since ancient times, making weapons, tools, dishes and many other products from it. Initially, instead of zinc, which was discovered by scientists only 5 centuries ago, zinc-containing ore – galmey – was used to produce such an alloy. The tombak itself was invented in the 17th century. It is believed that the author of the chemical composition of this alloy was the Englishman Christopher Pinchbecker.
Thanks to its characteristics (first of all, its very beautiful color), tombac became known throughout the world in just a few decades. By the way, this alloy gained enormous popularity among scammers who passed off products made from it as gold, deceiving gullible citizens and earning a lot of money from it. Both before and now in everyday life and among specialists you can hear different names for tombak:
- princetal;
- chrysochalk;
- similor;
- oreid;
- Chrysorin.
Cold-rolled brass strip L90 - the material from which tombak products are subsequently made
Tompak, as is already clear from all of the above, is an alloy of copper and zinc, with the first element contained in tombak 88–97%, and the second (zinc) – no more than 10%. There is also such a material as semi-tompak. This is an alloy whose chemical composition is 10–20% zinc.
The amount of zinc in the composition of any brass, including tombac, affects not only the characteristics of the alloy, but also its color. Thus, with an increase in the content of this element in the composition of brass, the color of its surface changes from red to light yellow. Increasing the quantitative content of zinc in the chemical composition of tombac makes it possible to achieve:
- obtaining a noble golden color;
- improving anti-friction characteristics;
- good mechanical and technological characteristics;
- significant reduction in cost (when compared with the price of pure copper).
Chemical composition of copper alloys used to imitate gold
Tompak has a whole list of advantages, which include:
- exceptionally high corrosion resistance;
- increased wear resistance;
- good weldability with various materials (steel, non-ferrous and precious metals);
- high ductility (which makes it possible to use plastic deformation methods for processing tombak and engraving on the surface of products made from it);
- subject to enameling and gilding;
- non-magnetic.
This is interesting: Business for the production of metal structures
Tompak also has the following characteristics:
- hardness – up to 145 MPa;
- the temperature at which the alloy begins to melt is 10450 Celsius;
- friction coefficient: without the use of lubricant – 0.44; when using special type lubricants – 0.074;
- tensile strength - 440–520 MPa;
- the relative elongation that the material receives after breaking is 3%;
- material density – 8780 kg/m3.
The characteristics of L90 brass, which is called “tompak,” can be improved if various components are added to its chemical composition in small quantities. This minor modification allows us to obtain a material that optimally combines characteristics such as ductility, strength, good workability, attractive appearance and affordable price.
Modern manufacturers produce tombak in the form of rolled sheets or bars, which determines the options for further use of such material. You can find out exactly what elements and in what quantities are included in the chemical composition of the alloy by its marking. Thus, the letter “L”, which is necessarily present in the designation of tombak, means that this is brass. The numbers after this letter indicate how much base metal – copper – is contained in the alloy. In such brass, in addition to copper and zinc, there are necessarily impurities, the exact amount of which can be found out from special tables. It should be borne in mind that alloys in which copper is less than 90% (L85, L80, etc.) belong to the semi-tombac category.
Comparison by technical and user characteristics
Life time. The smooth inner surface of steel pipes, coated on the outside with an aluminum jacket, does not contribute to the deposits of lime and salt deposits.
The typical service life of bimetallic radiators is more than 20 years, and here their only rival is cast iron.
It is believed that due to unreliable paint and varnish coating and insufficient corrosion resistance of steel, the service life of steel appliances is lower and ranges from 15-20 years.
Heat transfer from radiators is a relative concept. Aluminum has a high heat transfer coefficient. The bimetallic section has a heat output of about 200 W. For steel sections of the same area, this figure is almost two times lower.
Properties
Tompak is an alloy containing zinc and copper, which allows it to be called a type of brass. The composition of this compound can vary greatly:
- the percentage of copper can vary from 88 to 97%;
- the percentage of zinc should be no more than 10%.
Many properties, including strength and color, are determined by the concentration of zinc . As the content of the latter increases, the color changes from red to a delicate shade of yellow, which is as close as possible to gold. Also in this case, a number of characteristics of the metal change:
- increasing anti-friction properties;
- improvement of chemical and technological features;
- lower cost of the alloy (compared to copper).
Tompak has a number of distinctive features:
- hardness is about 146 MPa;
- the melting point is at 10450 degrees;
- the friction coefficient in the absence of lubrication is 0.44, and with the addition of lubricants this figure decreases to 0.074;
- tensile strength is in the range of 445–525 MPa;
- relative elongation after rupture – 3%;
- density – 8780 kg/m3.
The described parameters of tombak can be improved by adding various components in not very large quantities. In this way, it is possible to obtain alloys with an optimal combination of main characteristics. This metal is produced in the form of sheets or bars. Markings are applied to the metal, by which its chemical composition can be determined. For example, the presence of the letter "L" indicates that it is brass. From the numbers you can determine the amount of copper.
In addition to copper and zinc, it also includes other impurities; their presence and quantity can be found in specialized tables.
Notes
- ↑
A dictionary of arts, manufactures and mines: containing a clear exposition of their principles and practice Robert Hunt (ed.), D. Appleton & Co.: 1856: pp243 - ↑
Bank of Russia. A variety of Bank of Russia coins in denominations of 10 and 50 kopecks, model 1997. — (coins of the 1997 sample, coated with tombac, have been issued since 2006, previously they were made of brass). Retrieved January 29, 2009. Archived from the original on June 2, 2012.
| This is a draft article on chemistry. You can help the project by adding to it. |
| Coin metals | |
| Metals | Aluminum (Al) | Iron (Fe) | Gold (Au) | Copper (Cu) | Nickel (Ni) | Tin (Sn) | Palladium (Pd) | Platinum (Pt) | Silver (Ag) | Lead (Pb) | Chromium (Cr) | Zinc (Zn) |
| Alloys | Akmonital | Aluminum bronze (CuAl) | Bronze (CuSn) | Kolyvan copper (CuAuAg) | Brass (CuZn) | Copper-nickel alloy (CuNi) | Cupronickel (CuNiFeMn) | Nickel silver, nickel silver (CuZnNi) | Stainless steel (FeCrNi) | Nickel Bronze (CuSnNi) | Nickel-iron alloy (NiFe) | Nickel-zinc alloy (NiZn) | Potin | Northern gold (CuAlZnSn) | Steel (Fe) | Sterling (AgCu) | Tompak (CuZn) | Chrome steel (FeCr) | Cast iron (Fe) | Electrum, electron, electrum (AuAg) |
| Groups of coins | Bimetal | Billboard | Bronze | Copper | Iron | Gold | Palladium | Platinum | Silver | Siberian |
| Metal groups | Coin group (copper subgroup) | Noble metals | Platinum group |
| see also | Paper money | Polymer money | Money paper | Leather rubles | Stamps-money | Coinage | Notgeld | Symbols of precious metals |
tompak, tompak 2 mausym, tompak all series, tompak zhekpe-zhek, tompak kazaksha cinema, tompak costasu, tompak mektep turals, tompak cartoon, tompak cartoon, tompak series
Brass is an alloy based on the metals copper and zinc. The zinc content in the alloy can be from 5 to 45%. Zinc is cheaper compared to copper, for this reason its introduction into the alloy not only improves the mechanical, anti-friction and technological properties, but also reduces the cost of brass.
Brass can be called the most outlandish alloy of antiquity. In the Roman Empire, production of the alloy began in the 1st century BC. Among precious metals, brass ranked third after silver and gold. In the East, the alloy has been known since the 8th century. The source of copper, lead and silver is considered to be the Anarak mine, which is located in northern Iran. There is evidence of the use of brass alloys in the 8th-9th centuries in the North-West Caucasus. Along the Silk Road, residents of the North Caucasus could buy brass from Asia Minor. In England in 1781, brass was made by alloying copper with zinc.
Read also: Forged clothes racks
Areas of application
Tombak has become widespread in various fields of human activity. Here are the most popular areas.
- Production of jewelry, ornaments, interior items and kitchen utensils . Tompak is valued in this area primarily as a material that imitates gold. Its noble color, pliability and high decorative effect make it an excellent solution for such purposes.
- The production of tableware is especially important in the countries of Central Asia, since here such household items are valued and held in high esteem. Brass does not tolerate contact with liquids, therefore dishes made from the raw materials in question are necessarily subjected to tinning - a technology during which a thin layer of tin is applied to the item.
- Modern industry also actively uses tombac. It is used to make wire for various purposes.
- The creation of technical parts is carried out in various ways. The most commonly used method is based on the principle of mechanical removal of material from the surface. Special turning and milling machines are used for processing. Due to the high relevance of this technology, many tombac blanks began to appear.
- There are a large number of organizations whose production activities are aimed at producing rolled products. The metal in this form is processed on turning equipment. Rolled tombac is characterized by different diameters, which cannot be said about the length and basic properties, which are standardized characteristics. Such features make it easy to select the necessary workpiece for future production.
The area of use of the metal largely depends on its resistance to corrosion.
Where is Tompak most often used?
Tombak is used for cladding steel, i.e. coating the surface of steel products with a thin layer of an alloy, which is used in the manufacture of charges and bullets for firearms and producing a steel-brass bimetal. The use of such bimetallic sheets saves stainless steel and non-ferrous metals, protecting the steel core from corrosion and destruction and giving the surface the desired properties. This technology can dramatically reduce the cost of the product. Bimetal is used for various elements of chemical equipment, where other coatings cannot cope with corrosion. Bimetallic wire is used for communication lines. Tombak is used for the manufacture of high-precision measuring and other equipment.
Due to its good resonator properties, the bells of brass wind musical instruments are made from the alloy. It is used to make:
- artistic products (paintings made using metal chasing technique);
- radiator tubes and drawn pipes with a diameter of up to 30 mm;
- insignia;
- accessories;
- cigarette cases and cigarette holders;
- some types of medals and coins.
For example, Russian coins in denominations of 10 and 50 kopecks. School Gold medals are made from tombac and then gilded. The bronze medals of the 1980 Summer Olympics in Moscow were made of tombac. In 1942-1943, Canada issued tombac coins in denominations of 5 cents.
Due to its beautiful color and ease of processing, tombak is widely used by jewelers in the manufacture of costume jewelry. The alloy is used to make dishes, figurines, candlesticks, and in interior decoration and furniture. In Eastern countries, elegant thin-walled waterware, jugs, bowls, and trays are made from tombac, which is decorated with unique patterns, thereby creating works of art. The dishes are protected from the destructive effects of water on the alloy by tinning with tin, which allows the artistic beauty of the products to be preserved for a long time.
Steel radiators
Steel radiators are divided into two types according to their design: tubular and panel.
Production methods
Methods for obtaining tombak have expanded significantly since its discovery, but the essence of each of them comes down to a single scheme.
- The composition is heated in an electric oven to 1300–1400 degrees. With such heating, silicate is released, floating to the surface of the alloy, which makes it possible to remove it from there without unnecessary difficulties.
- The resulting metal is poured into a container and purged with oxygen. This stage requires the use of special equipment. This treatment provokes the release of thermal energy in large quantities and triggers a chemical reaction.
- At this stage, copper is formed, which contains many impurities, which reduces its properties.
- The composition is subjected to electrical cleaning using acidified copper sulfate.
- Zinc is added to molten copper to produce a strong alloy with high corrosion resistance.
The process of obtaining tombak is complex, labor-intensive and energy-consuming. This explains the high cost of the metal, which is still much lower than the price of gold.
Production methods
Today, tombak can be produced using a variety of technologies. The most widely used option is the use of an electric oven, which heats the composition to 1400 degrees Celsius. When exposed to such a temperature, silicate is released, which floats to the surface and is removed.
Among other features, we note the following:
- The result is base metal, which is poured into a special container. There are quite a large number of different devices whose purpose is to release tombak. The base composition must be heated to a temperature of 1400 degrees Celsius. In this case, certain safety rules must be observed.
- The next step is to flush the resulting composition with oxygen. Special equipment is also used for this. The purging procedure leads to the active release of thermal energy and a chemical reaction.
- As a result of a chemical reaction when exposed to oxygen, copper is formed. It is characterized by the fact that it has a large number of various impurities, due to which the properties are significantly reduced.
- Next, the composition is electrically cleaned using special acidified copper sulfate.
- Zinc is introduced into the resulting straightened copper. This material increases strength and corrosion resistance.
The above information indicates that the process of obtaining such a composition is quite complex and time-consuming. That is why the cost of tombak is quite high, but much less than the cost of the precious metal.
Do not forget that tombac is an alloy of copper and zinc. This composition has very attractive characteristics and is used in the creation of various high-precision measuring and other equipment. In addition, decorative characteristics are highly valued. At a certain ratio of the main components, the alloy resembles gold, but the properties are significantly different.
Methods of obtaining
The technology for producing brass involves the processes of the copper and zinc industries, as well as the processing of recyclable materials. The raw materials for the production of alloys are blanks of copper, zinc and other metals for the production of multicomponent alloys. We also use our own production waste and secondary raw materials. All blanks are manufactured in accordance with GOST.
For smelting brass, various types of smelting furnaces are used, which are used for smelting copper alloys. The most effective are electric induction low-frequency furnaces with a magnetic core. Melting is carried out under exhaust ventilation, since some elements of the alloy evaporate intensely and can harm human health. It is undesirable to overheat the alloy, due to the likelihood of some components igniting in air. Pure and recycled metals are used as charges for smelting brass.
The raw materials are first prepared and the ovens are cleaned. Copper heated to red heat is placed in a furnace, and then lumps of zinc are added. When melting copper-zinc alloys, the significant oxidation of zinc is taken into account. To reduce oxidation, a number of measures are carried out. To make multi-component alloys, copper is added first, followed by the remaining components carefully.
The homogeneous mass is poured into molds to produce foundry brass. The result is flat and round ingots. After casting, wrought alloys undergo a deformation procedure. The resulting products differ in the degree of hardening and aging, as well as the hardness of the material. Preliminary heat treatment of workpieces significantly increases the strength and corrosion resistance of brass.
TOP 6 ways to clean tombac and remove oxide from the surface of tombac products
Although brass alloy of any grade is highly resistant to corrosion destruction, it is even called an “eternal metal”, over time an oxide film forms on the surface of products made from it. It darkens, the surface becomes dull. If the product is often in contact with water, the oxidation process is especially active. There are several effective ways to remove oxides, return the product to its original appearance, add beauty and shine.
Before using one of the methods, you need to make sure that the product is not coated with a brass alloy, but is made entirely of it. For such a check, it will be enough to use a magnet. Tompak and semi-tompak are non-magnetic alloys, so a magnet will not be attracted to the product. If the product is only coated with an alloy, there is no need to use abrasive substances when cleaning so as not to damage the surface layer.
First way
Prepare a solution consisting of 3 liters of water, a small tablespoon of salt (25 g) and a glass of ordinary vinegar. The product should be placed in this solution and boiled until the surface is completely clean. As necessary, add water to the original volume. After cleaning, the item is washed with running water and dried.
Second way
The item that needs to be cleaned is carefully placed for several hours in a solution consisting of 10 liters of water and 200 ml of oxalic acid. To make it, you must use plastic containers. Use this volume of solution to clean large products. When cleaning small items and the need to use less solution, you can proportionally reduce the volume of components. This method is potentially dangerous to the skin and eyes, therefore, if used, it is necessary to use rubber gloves and a respirator as personal protective measures. After cleaning, the product must be thoroughly rinsed under running water and rubbed with a dry cloth.
Products with enamel, glass inserts, other metals, paintings and other artistic elements are not recommended to be cleaned using these methods.
Third
A solution containing water and soap is required. You also need acetone. A cotton swab or disk is moistened in acetone and the product is thoroughly wiped with it. After such treatment, it is washed with soapy water until the original shine of the surface is completely restored and be sure to wipe dry.
Fourth method
The simplest one is to wipe the surface of the product to be cleaned with half a lemon or lime, which is first dipped in table salt. After such cleaning, the surface can be polished with regular toothpaste and a cotton cloth as a gentle abrasive polishing compound. Then you need to rinse the item in running water and wipe dry.
Fifth method
You need to buy a cleaning agent for non-ferrous metals in retail chains. Most often it is sold in the form of tubes with cream or paste, and apply it strictly according to the instructions.
Sixth method
Polish the product using GOI paste. This product is called the paste of the State Optical Institute and was developed about 80 years ago. To clean and polish the product, apply the paste to a soft cloth. A few drops of spindle oil are applied to better dissolve the paste, and then the product is polished with slow movements. After polishing, the item is washed to degrease and wiped dry.
Reanimation of the surface from oxide
Due to their wear resistance, brass and all its types are called “eternal metal”. But this does not mean that products made from these alloys do not need to be looked after. They, like silver, darken, especially due to contact with water. It’s easy to fix the situation; let’s look at several ways to clean brass.
For the simplest case, you will need acetone, soap, water and a cotton pad. Prepare a weak soap solution and soak the cotton wool in acetone. Wipe the product thoroughly with a disc and then wash it in the solution. All that remains is to dry it and enjoy the magnificent view. The second method is also available to any housewife. You will need a metal container with a minimum capacity of 3 liters, water, 250 ml of simple vinegar and 25 g of salt. Mix all the ingredients, place the brass product in the solution and place the pan or bowl on the fire. Bring to a boil and add clean water if necessary. We continue until the brass item is clean.
The following method can be dangerous to the skin and eyes, so it requires caution. To clean tombac using this method, mix 10 liters of water and 200 g of oxalic acid in a plastic container. This method is used for processing large products. Be sure to follow safety precautions. Wear rubber gloves and a respirator. Carefully place the product in a container with the solution and leave it there for several hours. Then very carefully remove it and rub it with a dry cloth.
This is interesting: At what angle to sharpen drills for metal. Sharpening options that can be done by hand
You can also clean tarnish with lemon. Cut the fruit and immerse it in table salt, and then rub the surface to be treated. If you also need to polish, then a simple toothpaste will come in handy. Rub it in with a cotton cloth, and then rinse the element in clean running water.
Before cleaning, make sure that the product is actually made of brass and not covered with it. To do this, just bring a magnet. Tompak will not react at all. If the item is just coated with a thin layer of alloy, do not use abrasive substances, as this can easily damage the surface layer.
First way
Prepare a solution consisting of 3 liters of water, a small tablespoon of salt (25 g) and a glass of ordinary vinegar. The product should be placed in this solution and boiled until the surface is completely clean. As necessary, add water to the original volume. After cleaning, the item is washed with running water and dried.
Second way
The item that needs to be cleaned is carefully placed for several hours in a solution consisting of 10 liters of water and 200 ml of oxalic acid. To make it, you must use plastic containers. Use this volume of solution to clean large products. When cleaning small items and the need to use less solution, you can proportionally reduce the volume of components. This method is potentially dangerous to the skin and eyes, therefore, if used, it is necessary to use rubber gloves and a respirator as personal protective measures. After cleaning, the product must be thoroughly rinsed under running water and rubbed with a dry cloth.
Products with enamel, glass inserts, other metals, paintings and other artistic elements are not recommended to be cleaned using these methods.
Third
A solution containing water and soap is required. You also need acetone. A cotton swab or disk is moistened in acetone and the product is thoroughly wiped with it. After such treatment, it is washed with soapy water until the original shine of the surface is completely restored and be sure to wipe dry.
Fourth method
The simplest one is to wipe the surface of the product to be cleaned with half a lemon or lime, which is first dipped in table salt. After such cleaning, the surface can be polished with regular toothpaste and a cotton cloth as a gentle abrasive polishing compound. Then you need to rinse the item in running water and wipe dry.
Fifth method
You need to buy a cleaning agent for non-ferrous metals in retail chains. Most often it is sold in the form of tubes with cream or paste, and apply it strictly according to the instructions.
Sixth method
Polish the product using GOI paste. This product is called the paste of the State Optical Institute and was developed about 80 years ago. To clean and polish the product, apply the paste to a soft cloth. A few drops of spindle oil are applied to better dissolve the paste, and then the product is polished with slow movements. After polishing, the item is washed to degrease and wiped dry.
What is a semi-tompak?
Semi-tompak should be included in a separate category. The differences lie in the concentration of zinc, which increases to 10-20%. Due to this, the basic properties of tombak change. The key features are the following:
- The product takes on a more yellow tint.
- The degree of ductility decreases and refractoriness increases.
- The material is often used to make technical parts.
This alloy is found much less frequently in comparison with tombac. This is due to the fact that an increase in the amount of zinc causes a large number of deficiencies to appear.
Corrosion resistance
In many ways, the material in question is popular due to its high corrosion resistance. Thereby:
- Tompak alloy is used in the manufacture of various decorative products that do not lose their properties over a long period. An example is tableware or inexpensive jewelry.
- The service life of products is significantly extended. Corrosion not only spoils the decorative qualities, but reduces the basic properties.
- High corrosion resistance can be achieved by using copper as the basis of the composition. This material does not react to moisture and some chemicals.
In general, we can say that the scope of application of tombac largely depends on the corrosion resistance indicator.
However, over time, due to exposure to high humidity, an oxide film appears. It significantly reduces the decorative qualities of the resulting product.
How to remove an oxide layer from the surface of a tombak product?
Despite the high corrosion resistance of tombac, such a metal is characterized by the fact that during prolonged use an oxide film appears on the surface. It is water that causes this problem. There are several different methods for removing the oxide film from the surface. An example is the following:
- The first method involves using water and soap. In addition, the composition includes acetone. The procedure involves wetting a cotton pad, which is used to wipe the surface. After carrying out such a procedure, the product must be washed in soapy water.
- The second involves the use of water, salt and table vinegar. In this composition, the product is boiled until the oxide film disappears from the surface.
A more dangerous, but also effective method of cleaning the surface involves using a mixture of water and oxalic acid. This mixture is used exclusively with rubber gloves, since the substance does not come into contact with the surface of the skin.
Rental products
The production of parts can be carried out in a variety of ways; the most widely used method is mechanical removal of material from the surface. Processing can be carried out using turning or milling equipment. The fairly wide spread of this technology was determined by the appearance of a large number of blanks.
The production activities of many organizations are aimed at producing rolled products. It is processed on turning equipment and can be characterized by different diameters. The length and basic properties are standardized, which allows you to select the appropriate blank for the production of the required product.
What is brass
The main components of brass alloy are copper and zinc. The proportional components of these metals may be different. The amount of zinc varies. Its minimum value is 20%. The maximum reaches 50%. At the same time, the alloy changes its color: it can be golden, yellow or green.
The percentage of zinc is so important that it can change the characteristics of the material. This refers to its ductility and hardness.
Structure and composition
The composition of the alloy is formed from the phases:
- Alpha phase. Zinc content up to 35%
- Beta phase. The presence of zinc is up to 50%. The composition also includes tin - 6%.
In some cases, a single alpha phase is present. Depending on changes in the percentage composition of the main components, the structure of brass can simultaneously consist of 2 phases - alpha and beta.
The chemical composition of brass, in addition to copper and the main alloying element zinc, includes additives. These include alloying elements: aluminum, iron, manganese, lead, silicon, nickel. They make up a small percentage of the compound. Each of them affects the characteristics of the material.
Properties and characteristics
The main quality in the characteristics of brass is its corrosion resistance. But it also has other properties:
- The ability of the alloy to withstand aggressive environments, especially after coating the surface with varnish.
- Strength of brass.
- Plasticity of the alloy.
- The ability of the material to be processed by pressure. The process is carried out both hot at high temperatures and cold.
- The alloy can be subjected to resistance welding and soldering.
- Thermal conductivity, which increases with increasing percentage of copper.
- Melting point, which is 880–950 degrees. With less zinc added, the melting point decreases.
- The material has non-magnetic properties.
The main factor in the hardness and ductility of the joint is zinc. An increase in its quantitative content is directly related to an increase in strength characteristics. Plasticity increases only up to a quantitative zinc content of 36%. With a subsequent increase to 45%, this indicator decreases.
In order to increase the hardness of the alloy, a heat treatment called cold hardening is carried out. It helps not only to increase the strength index, but also relieves internal, structural stresses.
Alloying additives affect the performance characteristics. Their influence is indicated in the table:
| Name of alloying element | Effect on brass characteristics |
| Silicon | Its high presence leads to a decrease in the hardness of brass. |
| Lead | Improves anti-friction properties. |
| Manganese, aluminum and tin | Increases resistance to tearing. Corrosion resistance is increasing. |
| Nickel | Reduces the risk of material cracking. The alloy acquires a peculiar color. This connection is called “white brass”. |
| Arsenic | The material has the ability to work in liquid, fresh media. |
Marking
There are 2 types of alloys:
- Two-component. The main components are copper and zinc. They are marked with the letter L. Next are numbers indicating the amount of copper in percent. L60: contains 60% copper, and the remaining 40% zinc.
- Multicomponent. In addition to the main components, alloying elements are added. Also in front is the letter L. Then follows a list of additives. At the end, numbers are written through a dash indicating the percentage of each component. The amount of zinc is not indicated, but calculated. For example: Brand LAZhMts66-6-3-2 has 66% Cu, 6% Al, 3% Fe and 2% Mn. By calculation, the amount of zinc is determined to be 23%.
Sources
- https://vplate.ru/metally-i-splavy/osobennosti-i-sostav-tompaka/
- https://lux-stahl.ru/metally-i-splavy/tompak-chto-eto-takoe.html
- https://moy-instrument.ru/masteru/material-tompak-chto-eto.html
- https://tutmet.ru/tompak-listovoj-sostav-splava-foto.html
- https://intehstroy-spb.ru/spravochnik/tompak-pochemu-ego-putayut-s-zolotom.html
- https://SevenTools.ru/metally/tompak-ili-bimetall-dlya-hromirovannogo-stvola.html
- https://stankiexpert.ru/spravochnik/materialovedenie/tompak.html
- https://martensit.ru/latun/tompak/
Basic properties of brass
Brass lends itself well to pressure processing. Mechanical properties are relatively high, corrosion resistance is satisfactory. If we compare brass with bronze, then their strength, corrosion resistance and anti-friction properties are less. They are not very stable in air, in salt sea water, carbon dioxide solutions and solutions of many organic acids.
Brass has a beautiful color and, compared to copper, has better corrosion resistance. However, as the temperature increases, the corrosion rate also increases. This process is most noticeable in thin-walled products. Corrosion can be provoked by: humidity, traces of ammonia and sulfur dioxide in the air. To prevent this phenomenon, brass products are subjected to low-temperature firing after processing.
Almost all brasses, when the temperature drops (to helium temperatures), remain ductile and do not become brittle, which makes it possible to use them as a good structural material. Due to the higher recrystallization temperature (300-370°C) than copper, the creep of brasses at high temperatures will be less. At average temperatures (200-600°C), the phenomenon of brittleness occurs, since impurities insoluble at low temperatures (for example: lead, bismuth) form brittle intercrystalline layers. As the temperature increases, the impact strength of brass decreases. Compared to copper, the electrical conductivity and thermal conductivity of brass is lower.
Read also: How to take electric meter readings day and night