Many people believe that any metal can be protected with a special oxide coating that will prevent corrosion. However, there is a special type of corrosion called pitting that affects metals with a protective coating. In most cases, pitting corrosion affects only the upper oxide layer of the metal, and penetrates deeper rather slowly.
But how exactly does pitting rust occur? Is it true that corrosion exists on stainless steels? Below we will find out the answers to these questions.
External manifestation of pitting corrosion
There are various forms of destruction of metals. Pitting or pitting is one of these forms, which is localized defects on the surface of a metal. Most often, pitting corrosion occurs on stainless steel, aluminum and its alloys, titanium, nickel and occurs when the passive state of the material is partially disrupted.
Pitting is quite dangerous for metal, despite the small size of its manifestations. The rest of the surface continues to remain in normal external condition, and only in some places white or reddish small dots, ulcers, and small stripes appear.
Their appearance is deceptive and their depth is usually considerable, yet the user rarely notices them at an early stage of development.
Causes of rust formation
The main reason for the formation of corrosion is improper processing and production technology of the material. Because of this, microparticles remain in the material, which subsequently lead to deformation and damage to the integrity of the product.
Most often, mill scale remains in the material, which provokes corrosion.
Due to the regular external influence of various factors on the surface of a metal object, pitting corrosion often begins to appear. It destroys the outer protective layer and forms ulcers throughout the area.
Pitting or ulcerative lesions on products form very quickly on an uneven, rough surface. They quickly affect the entire area of the metal, worsening its properties. When interacting with salt water, acid or other aggressive substances, the item quickly rusts and becomes unusable.
Reasons that initiate pitting
Often the prerequisite for the appearance of pitting corrosion is a violation of metal production technology. For example, if casting rules are not followed, microimpurities and inclusions appear in steel that change the normal structure. Low-quality metal may be too porous or residual scale may appear in it - this also contributes to the occurrence of pitting.
Pitting also occurs when steel and other metals are used in an aggressive environment: solutions containing oxidizing agents and activating anions (hydrochloric, nitric acids, sea water, chloride compounds).
Other causes of pitting corrosion are:
- mechanical impact leading to chips, scratches and causing damage to the outer protective film;
- excessive internal stress of the metal;
- operation of the product at high temperatures.
Pitting corrosion is more likely to appear on rough stainless steel than on smooth, polished steel, so uneven surface texture is also considered a risk factor.
How to protect metal
We looked at what causes pitting corrosion. By understanding the peculiarities of such a process, it will be possible to determine how to deal with it and ensure the protection process.
To combat such a problem, it will be possible to use a passivation process.
It is based on the use of a special solution, which contains two acids - citric and nitric. You can also use additives to greatly enhance the process.
The goal that is set when passivation is used is that the corrosion process can become slower or stop.
There are 3 methods to protect yourself from the causes of pitting corrosion.
These include:
- Elimination of defects . The use of modern methods allows us to eliminate most types of defects that may appear on metal. Polishing and other means are used to correct unevenness. This reduces the risk of corrosion.
- Polishing . Helps eliminate roughness. This prevents corrosion products from accumulating. Natural protective films may appear on smooth metal.
- Use of chrome plating . It allows you to protect the material from contact with aggressive environments. Galvanizing is used - this is the approach that helps improve the overall quality of protection.
Our company is always ready to create protection against damage to any types of metal structures and steel products.
Features and development pattern of pitting corrosion
Pitting has a high flow rate. If you do not get rid of minor defects in time, the product may rust through. The higher the temperature at the location of the metal, the faster it will rust.
Pitting corrosion develops in three stages:
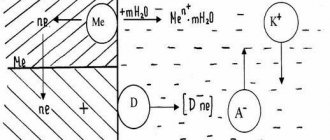
- The first stage is emergence. Typically occurs in areas where protection has been compromised, where the passive film on the metal surface has been torn, or where there is material heterogeneity. After oxygen is displaced by activator ions, the oxide layer is destroyed.
- The second is the growth of pitting. It obeys the laws of electrochemical reactions. Due to the dissolution of the oxide film, the anodic process intensifies at the site of pitting corrosion, while the normal surface becomes a cathode.
- The third is diffuse expansion. At this stage, the corrosion element moves deeper, and new rust spots may form nearby.
In some cases, pitting stops developing at the second stage and moves to the repassivation stage. This happens when the reaction shifts towards passivation, for example, when the acidity of the medium changes. If pitting corrosion has progressed to the stage of diffuse growth, it can no longer enter repassivation.
What is pitting
So, pitting is a type of corrosion in which so-called pitting will appear on the metal surface. Such corrosion will affect copper, iron, aluminum alloys, as well as those based on chromium and others. Pitting type corrosion is possible even on stainless steel.
Pitting will usually affect various metal structures that will come into contact with salt water (usually various areas near the shore). This is due to the fact that for the reaction to take effect, an excess of activator ions is required, which will displace oxygen from the protective oxide film - and such substances are released in large quantities in water. Also note that pitting usually begins on the top layers of the oxide film at first, but as rust progresses, it may begin to penetrate the entire metal. This type of corrosion usually occurs when two factors are combined.
Pitting shape
From the photo you can see that some elements have the correct shape, others are incorrect in appearance. The exact shape depends on the voids in the crystal lattice that formed during pitting initiation. Typically, irregular pitting corrosion occurs on plain (carbon), low-alloy steel and stainless steel, while correct pitting occurs on aluminum and various alloys. In addition, the classification of pittings by shape looks like this:
- hemispherical, with a shiny, polished bottom;
- polyhedral;
- faceted, including those interconnected;
- in the form of complex polyhedra;
- pyramidal;
- prismatic.
Polished (hemispherical) elements are often found on aluminum, tantalum and titanium, as well as on cobalt and nickel alloys.
Sensitization of stainless steels and corrosion of welds
This type of corrosion damage is caused by the separation of individual alloy crystals from the surface of the part. It is caused by the influence of working environments saturated with metal salts. In such environments, calcium salts are wedged between separated crystals, and the growth of carbide crystals leads to further destruction of the crystal structure.
Welded seams are susceptible to this influence, during which the welding technology was violated. Sensitization of areas of container shells is also possible. It's called knife corrosion and spreads in narrow stripes.
The crystal structure, weakened by sensitization, is especially susceptible to galvanic influence. Under the influence of induced potentials, the corrosion rate increases many times.
To prevent such negative processes, the method of passivation of stainless alloys and welds is used.
Pitting classification
Pitting corrosion is classified not only by shape, but also by other characteristics: size, specifics of its development.
By size
Depending on the exact composition of the metal, environmental conditions (temperature, acidity), the size of pitting corrosion can be different:
- microscopic (micropitting) – less than 0.1 mm;
- conventional (pitting) – 0.1-1 mm;
- significant (ulcer) – more than 1 mm.
According to the specifics of development
Pitting can be superficial, open or closed. Surface corrosion elements develop intensively horizontally, without affecting the deeper structures of the metal. They cause the appearance of clearly visible shallow potholes. Open pitting is visible to the naked eye or at a small size when magnified with standard optical equipment. This type of rusting often turns into complete rusting if a lot of pitting begins to appear on the surface.
Closed corrosion is considered the most dangerous in terms of the further safety of metal products. It is impossible to examine it without instruments, so the elements grow deeper into the metal, remaining unnoticed for a long time. It is the closed pittings that cause the formation of holes. If the initial signs of corrosion are not removed in time, the product will become unusable.
Stages of pitting growth:
1) The initiation of pitting occurs in places of defects in the passive film (scratches, breaks) or its weak points (if there is inhomogeneity of the alloy) when a certain potential is reached - the pitting potential (φpo). Activator ions displace oxygen adsorbed on the surface or, upon interaction, destroy the oxide protective film.
2) Pitting growth occurs by an electrochemical mechanism, due to the intensive dissolution of the passive oxide film. Due to the active dissolution of the film, the anodic process intensifies in the pitting itself (activation growth of pitting). Over time, when the pitting is sufficiently expanded, the activation growth slows down, and the diffusion mode of pitting growth begins.
3) Sometimes the growth of pitting stops and the stage of repassivation begins. The main reason for repassivation can be considered a shift in the surface potential in the negative direction, i.e. side of passivation. Pitting with a diffusion growth mode (gradually, steadily growing pitting) cannot go into the repassivation stage.
Methods of protection against pitting
There are a number of modern methods for preventing corrosion, and many of them are already used at the production stage of the car. However, due to long use and constant contact with aggressive reagents, old machines are susceptible to rust. Pitting often occurs on various car parts: bearings, gear teeth, and rust spots on the body are considered a common occurrence.
Pitting corrosion is often found on household items, including stainless steel. To protect metal, you can use mechanical and chemical methods, some of which are suitable for independent use.
Mechanical method
This method includes tips for removing existing rust using grinding, laser processing, as well as mechanical application of barrier coatings (including paints). The choice of coating type depends on the type of metal and its operating conditions. The technique of galvanizing or nickel plating is usually used, but in industrial settings chrome plating, plating with copper, silver, aluminum, tin, and cadmium is also practiced. The created film isolates the metal from the environment and prevents it from coming into contact with acids, oxygen, and chlorine, thereby extending its service life.
There are kits on sale for do-it-yourself galvanizing of metal. First, the part is cleaned of existing rust by processing it with converters. After half an hour, the products are washed off, the product is cleaned, polished, a layer of a special solution is applied and an electrode with a zinc tip is connected. After a certain time, a thin zinc film will be created on the metal surface, which will prevent rust from further destroying the material.
Chemical method
The main chemical method of getting rid of corrosion is the elimination of a closed system with solutions of alkalis, sulfates, and chromates. The principle of operation is to reduce acidity and shift the reaction towards alkaline, in which corrosion processes stop. It is only important to control the release of hydrogen, since this element itself increases the risk of pitting.
Unfortunately, it is impossible to completely eliminate the risk of pitting corrosion in everyday life. There is only a chance to weaken the influence of risk factors. It is better to use the product correctly right away and prevent the acidity of the environment from increasing, rather than extending its service life by several years.
Methods for protecting metals and alloys from corrosion
Protection of metals and alloys from pitting corrosion:
- Electrochemical protection. This type of protection is often used in conjunction with the use of inhibitors.
- Since passive alloys are most susceptible to pitting formations, the best solution would be to minimize their size in the composition of the alloys or completely replace them with another material that is more resistant to destruction.
There are substances whose introduction into the alloy increases its anti-corrosion resistance (for example, silicon and chromium).
- The use of inhibitors that, in a closed system, suppress or delay the development of destructive processes due to chemical and physicochemical influences (sulfate-based substances, alkalis, nitrates).
- Applying a protective anti-corrosion coating to the previously cleaned and prepared surface of the element.
It is important to understand that the presented methods of combating pitting corrosion of metal are only possible in production or at the initial stage of creating an alloy for a structure or part.
Thus, the presence of pittings does not put an end to the performance of the structure if they were noticed in time. You should always carefully monitor the condition of the products included in the work, carry out technical inspections during the process, and do not skimp on high-quality anti-corrosion protection for metals.
What is pitting corrosion of stainless steels - types and methods of protection
GOST No. 5272 of 1968 defines various types of destruction of metals (alloys) and classifies them by type and type. Pitting corrosion is not an entirely correct name, if you focus not on the commonly used terminology, but on the standard. Its correct name is dotted. The regulatory document explains that pitting is a type of localized corrosion.
Stainless steel is a general definition of alloys, which are divided into 3 groups. They differ in the specificity of their application and the prevalence of those characteristics that are most important in each specific case. Next, we will mainly talk about the most common modification of products - corrosion-resistant steel.
External manifestation of pitting corrosion
It is expressed in pinpoint lesions of alloys (including stainless steels) and metals. Pitting corrosion begins at the surface of the sample and gradually spreads deep into the structure, causing cavities (ulcers) to appear in the material. Most often it appears in places of various defects in stainless steel.
Reasons that initiate pitting
- Mechanical impacts on metals, resulting in the formation of scratches and dents on stainless steel. The heterogeneity of the structure of stainless steel is one of the causes of pitting. Natural processes - internal stresses, various micro-inclusions and a number of others. Damage to the protective (anti-corrosion) coating of stainless steel. Failure to comply with the technology of production and processing of the alloy - increased porosity of the structure, residual scale. Surface condition of a stainless steel sample. Its roughness increases the risk of pitting. Aggressive environments. Steel is negatively affected by sea water, acidic environments, and so on.