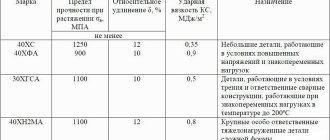
Among the large number of natural resources that people use to create comfortable living conditions, there are minerals with metal impurities. The extraction of iron constituent elements from mineral formations has created an entire industry, without which it is impossible to imagine the development of scientific and technological progress and the life of modern man. Iron ore has been considered the main resource in metal mining for many centuries.
Classification
Iron ores are classified according to a number of characteristics.
First of all, the most important factor in economic terms is the percentage of iron. Therefore, ores are divided into a number of types:
- Rich – the content of useful mineral exceeds 50%.
- Conventional ores contain 25 - 50% iron.
- Iron-poor raw materials do not have a significant amount of iron. It contains no more than 25%. That is why such ores are called poor ores.
As for the chemical composition, these are mainly various compounds of iron with oxygen, water and carbon, found in nature in the form of:
- brown iron ores – limonites,
- magnetic iron ores - magnetites,
- red iron ores – hematites,
- spar iron ores – siderites.
Iron-bearing deposits usually contain the following types of ores:
- Apatite-magnetite, contained in carbonatites.
- Goethite-hydrogoethite, located in weathering crusts.
- Magnetite and magma-magnetite in the skarn environment.
- Magnetite-hematite, found among iron quartzites.
- Martite and martite-hydrohematite are especially mineral-rich ores in iron quartzites.
- Titanium-magnetite, as well as ilmenite-titanomagnetite, located in mafic and ultra-mafic rocks.
To complete the picture, it should also be noted that deposits of this type of minerals are formed as a result of:
- high temperature exposure – magmatic deposits;
- weathering of mountain rocks and sediments – exogenous;
- sedimentary activity, subsequently subjected to significant pressure and temperature.
What does iron ore look like and what is it?
Iron as a chemical element is included in the composition of many rocks, however, not all of them are considered raw materials for mining. It all depends on the percentage composition of the substance. Specifically, iron ore refers to mineral formations in which the volume of useful metal makes its extraction economically feasible.
The extraction of such raw materials began 3000 years ago, since iron made it possible to produce higher-quality durable products in comparison with copper and bronze (see copper ore). And already at that time, craftsmen who had smelters distinguished the types of ore.
Today the following types of raw materials are mined for further metal smelting:
- Titanium-magnetite;
- Apatite-magnetite;
- Magnetite;
- Magnetite-hematite;
- Goethite-hydrogoethite.
Iron ores
Iron ore is considered rich if it contains at least 57% iron. But developments can be considered feasible at 26%.
Iron in the rock is most often in the form of oxides, the remaining additives are silicas, sulfur and phosphorus.
Factors determining the value of ores
The profitability of developing each specific field is explained by a whole set of conditions:
- The quantitative and qualitative composition of the main mineral, that is, again, the concentration of iron in the ore. It is clear that the higher it is, the better. This factor has a decisive influence on the yield of the final product and the smelting process itself. It is this that increases equipment productivity and does not require additional enrichment costs.
As for the deposit's reserves, the minimum required for a return on investment, according to economists' calculations, is 600 million tons. Smaller sizes do not cover the costs of creating the necessary infrastructure: production facilities, utility networks, roads, housing, public buildings.
- Also, the remaining composition of the ore is of great importance - that is, waste rock, which, depending on its qualities, can increase or decrease the yield of slag.
- The presence of impurities plays a very important role. If the useful ones improve the quality of the smelted metal, then the harmful components must be disposed of using complex technological methods or their adverse effects must be neutralized.
- The physical and metallurgical properties of the ore must also be taken into account. Dressability, strength, softening, lump size, moisture content - all these are factors that determine the potential value of iron ore deposits.
- In addition, the ability of the source material to recover - to release oxygen, which significantly speeds up the smelting process - is also important.
- One of the conditions that determine the economic feasibility of development is the depth of the ore body and its location, depending on the distance from developed economic regions. Overcoming these problems requires building roads and providing the field with human and energy resources.
Compound
The elements that make up iron ore are presented in the form of mixtures with mineral components, without pure iron or metallic impurities. They may be mixed with particles of limestone, clay, or other components formed as a result of eruptions or other natural phenomena. Most often, ores are found in the form of:
- siderite, iron spar;
- brown iron ore, which is called limonite, is characterized by lake or swamp origin;
- hematite;
- magnetite.
Iron ores can take the form of various compounds:
- salts;
- hydrates;
- just oxides.
Finding these useful constituent elements is a painstaking and time-consuming process. It is more difficult to extract metal impurities from minerals.
Depending on the amount of useful elements included in the raw material, monomineral and polymineral ores are distinguished.
If a monomineral resource includes only one valuable mineral, then in polymineral fossils, there may be more than two important metal components.
If there is one useful mineral, iron ore is called simple, and if several elements are present, such raw materials are classified as complex. The rare metal elements included in complex ore make the ore valuable and important for extraction and further use in mechanical engineering and instrument making. More than 80 chemical elements are extracted from ore and used in the production of various equipment.
According to the predominance of any of the mineral elements, the ore is divided into:
- native;
- phosphate;
- carbonate;
- sulfide;
- silicate;
- oxide;
- mixed.
Platinum and gold are extracted from native ores. The geochemical iron composition and properties of such minerals are associated with specific rocks. This connection contributes to the correct choice of the territorial location of deposits of gold and other valuable types of ores.
Depending on the industrial value of the iron elements, the ore can be disseminated or solid. The latter, in composition, has a higher ratio of valuable minerals to impurities or is a type of iron compound, and disseminated - includes from 20 to 60% of particles of various shapes that must be extracted from ore rocks.
The constant demand for metal products, over the years, adjusts the minimum percentage of valuable minerals in the ore, which is economically profitable to mine. If in the middle of the last century they developed deposits with subsequent processing of iron ore with a mineral content of at least 60%, today, thanks to modern equipment and the constant need for raw materials, ore with a metal content of 25-30% is used.
Extraction methods
The mining method is determined depending on the individual nature of the ore occurrence. The decisive factor, of course, is depth.
Open
As usual, if mineral resources are located not far from the surface of the earth (about 300 meters) and there is scope for carrying out a large amount of work to open and move soil, then they resort to creating a quarry. Powerful excavators move the rocks into dumps, and when they reach the lower layers of the deposits, they conduct a final analysis of the deposit for the percentage of iron content.
The final decision is made by the expert commission. In case of a positive result, the masses of seized rock are sent to metallurgical enterprises for further processing.
Closed
Although a significant amount of iron ore is mined by quarrying, sometimes it is necessary to resort to the construction of deep mines. This happens if the desired layers of minerals are located at a depth of about one kilometer. The process itself consists of laying a vertical shaft, from which horizontal drifts subsequently branch off.
Despite all its shortcomings (high cost of construction and danger of operation), this method is the most effective.
Also, in addition to these two methods, the method of borehole hydraulic extraction has recently found use. Its essence lies in the fact that water is supplied under significant pressure into the drilled well. As a result, the rock eroded by the jet moves upward.
Iron ore
How did metal ore deposits arise? Let's try to figure it out...
The Earth is known to have formed more than 4.5 billion years ago from particles of dust and gas left over from the formation of the Sun. This means that, theoretically, in the newborn Earth, all its constituent chemical elements should have been distributed evenly (well, except that the heavier ones tended to take place closer to the center). But today metals are smelted from ore - a rock that contains more of these metals than other minerals, and the ore, in turn, is found in natural accumulations called deposits. It turns out that not a trace remains of the original uniformity!
In fact, imagine that there are no deposits and all the elements of the periodic table are scattered throughout the earth's crust. In this case, one ton of rock would contain approximately 80 kg of aluminum and 50 kg of iron, and only 50 grams of such an essential metal for humans as copper would be found. Tin is even less - about two grams. It’s not worth talking about precious metals - in a ton of rock we could only find hundredths and thousandths of a gram. Can you imagine how difficult it would be to extract metals from such rock? And most importantly, it is unlikely that our ancestors would have even guessed about the existence of metals, which means we would still have lived in the Stone Age!
What processes cause the concentration of metals in certain places in the earth's crust? There are quite a lot of them, and nevertheless, they can be divided into two large groups: some are caused by underground heat, others by solar radiation.
Read: Natural gas - history of the use of fossil fuels
SUBSOIL ENERGY
Thanks to underground heat, molten magma exists in the bowels of the Earth and deep waters are heated. But once at the surface, they, of course, begin to cool. As a result of the solidification of magma, so-called igneous rocks are formed - most of the earth’s crust consists of them. However, for a deposit to form, it is necessary for a mineral with a high content of a particular metal to separate from the rest of the rock. This can happen if the melting point of that mineral is higher than the melting point of the parent rock. In this case, it crystallizes before the rest of the magma hardens and “sinks,” sinking to the bottom of the magma chamber. This is how deposits of chromium ores and platinum are often formed. Or, conversely, if the ore-forming mineral is more fusible, then it will solidify when all the rest of the magma has already solidified, and will fill the cracks between the masses of the main rock. This method is typical, for example, for the formation of titanium ore deposits. Finally, in some cases, liquid magma can separate into two immiscible components (much like milk separates into cream and skim whey), which then crystallize separately. This type of ore concentration is rare, but it has produced some very large copper-nickel ore deposits, for example in Norilsk.
Now let's talk about the role of water. Formed near the surface, it, under the influence of gravity, gradually seeps through cracks in the rocks deeper and deeper. And since the closer to the center of the Earth, the higher the temperature, the water begins to gradually warm up. At the same time, during its journey through the depths of the earth, water dissolves many different substances.
Read: Garnet stone
Due to the fact that hot water has a lower density than cold water, at a certain point deep water begins to rise back to the surface - and, accordingly, cool down.
With a loss of temperature, the solubility of minerals in water also decreases, and they begin to precipitate, that is, accumulate in the form of solid particles. It would seem - how many necessary minerals can water seeped into the depths of the Earth accumulate? However, deposits formed in this way, called hydrothermal, are widespread. This is how most deposits of lead-zinc, mercury, uranium ores, as well as almost all gold-bearing veins, appeared.
SOLAR WORK
Well, what if the rock is already close to the Earth’s surface and you can’t count on underground heat? In this case, deposits can be formed due to various mechanical and chemical processes, the energy source for which is sunlight. There are four types of such processes.
Firstly, water, seeping from the surface underground, dissolves various substances that can precipitate when physical or chemical conditions suddenly change. This is how many deposits of uranium, copper and silver ores were formed.
Secondly, deposits can also be formed due to the fact that water carries highly soluble compounds from the near-surface layers, which gradually accumulate: all the largest deposits of aluminum ores appeared in this way.
Read: Destruction of granite in nature
Thirdly, not only groundwater, but also surface water can contribute to the accumulation of certain minerals. In places where the speed of water flow drops sharply, for example, when the slope of a river decreases, the heaviest fractions of the particles transported by it are deposited on the bottom. This is how, for example, gold placers were formed, which caused the gold rush in California, Alaska, and Siberia.
Fourthly, the formation of deposits can occur as a result of the accumulation of sediment in water bodies.
For example, billions of years ago there was almost no oxygen in the Earth’s atmosphere, but the ocean waters contained a lot of dissolved iron. Then, as a result of the activity of the emerging blue-green algae, the atmosphere began to become saturated with oxygen, which dissolved in water and entered into a chemical reaction with the iron located there. Then the product of this reaction sank to the bottom and accumulated there - this is how the largest iron ore deposits occurred.
SCRAP METAL AND ECOLOGY
As you can see, the appearance of useful ore deposits on Earth occurred in different ways, over millions of years. But deposits are drying up much faster - modern mining methods devastate a mine in decades, or even years. If nothing changes, the supply of metal ores on Earth will quickly run out. Therefore, secondary raw materials are now becoming an important source of metals. More than a third of steel and aluminum and more than 20% of copper are produced from scrap metal. In addition to saving metal, recycling scrap metal can reduce energy use and seriously reduce the environmental burden.
Enrichment technology
Preparatory process
The preliminary stage of iron ore beneficiation is crushing and grinding. The purpose of these operations is to obtain a mass of the required size of pieces and particles, as well as to separate waste rock. Typically, this is done by screening (sifting) and classification (separation of particles by size by water flow) of the source material.
Main process
The enrichment process itself may include one of the following methods:
- Dry, wet or combined magnetic separation. The process is based on the different magnetic permeability of chemicals. In the case of wet separation, special electromagnetic drums remove minerals saturated with ferromagnets from the pulp. The dry method involves removing the magnetic fraction from the supplied charge using a rotating belt.
- The use of medium-density suspensions between iron and waste rock makes it possible to use gravity separation.
- The flotation method is based on the use of a special reagent that allows the formation of air-liquid metal foam, which is then removed and sent for further processing.
- The simplest enrichment method is washing. By itself, it is ineffective, so it is used in conjunction with other methods. But if the original rock is contaminated with clay or sand, you cannot do without it.
After the enrichment process, the concentrate is subjected to agglomeration and sent to blast furnace and then, if necessary, oxygen-converter smelting. Industrial waste can be used to extract rare or non-ferrous metals; sometimes they are used in the production of sand and crushed stone.
Helper process
During enrichment technology, it is often necessary to resort to auxiliary processes that ensure the removal of unnecessary fractions: dust, sludge, moisture. Thickening, sintering, filtering, drying make it possible to obtain a concentrate of the required readiness for subsequent use.
Iron ore reserves in the world
After the grandiose accumulation near Kursk, the largest phenomenon among similar ones on the world geographical map is the strip of iron deposits of the Krivoy Rog deposit in Ukraine.
Map of iron ore deposits in the world (click to enlarge)
Next in descending order is the resource of magnetites in the area around the Swedish city of Kiruna - a gift from volcanoes that lived in ancient times.
The wealth of the Lorraine iron ore basin is shared by three European countries - France, Luxembourg and Belgium.
In North America, large mines operate in Newfoundland, Belle Island and near Labrador City. In the South, places rich in ore were called Itabira and Karazhas.
Northeast India also has impressive ore reserves, and on the African continent it is mined in the Guinean city of Conakry.
The list of distribution by country looks like this:
Processed products
The main purpose of iron ore mining is to produce ferrous metals from it, obtained through the smelting process.
Steel
Well-known steel is a compound of iron (up to 45%), carbon (up to 2.14%) and a number of other chemical elements. Manganese, silicon, nitrogen, sulfur, oxygen, phosphorus. If necessary, chromium is added to its composition to increase heat resistance, or nickel to improve viscosity and anti-corrosion properties.
Depending on the carbon content C and alloying additives, steels are divided into:
- for low-, medium- and high-carbon;
- into low-, medium- and high-alloy.
According to their purpose, steels are classified as: heat-resistant, tool, structural, cryogenic and stainless steel.
A completely reasonable question arises: how is such a wide range of products obtained?
First of all, cast iron is smelted from the agglomerate under the influence of air in blast furnaces (this will be discussed in more detail in the next section of the article).
And then from cast iron through processing, which consists in reducing the carbon content, and in addition to it - sulfur and phosphorus (a significant amount of which worsens the mechanical properties of steel, increasing its fragility and brittleness), the final product - steel - is produced. These steel production processes are carried out using converter (Bessmer or Thomas), open-hearth or electrothermal methods. Depending on the thermophysical state of the source material (molten or solid) and the need for smelting certain types of steel, production methods can vary, sometimes complementing each other.
Cast iron
Cast iron is a high carbon (above 2.14%) iron alloy. It is due to this that it is characterized by increased fragility. It is produced by smelting processed ore in blast furnaces at a temperature of about 12000C.
Depending on its composition and production technology, there are light (white), gray, malleable, high-strength and pig iron. However, the latter is used only as an intermediate material for steel production.
Ore
Ferroalloy
One of the areas of modern metallurgy is the production of ferroalloys - compounds of iron with chromium, nickel, manganese, titanium and some other materials containing iron in small quantities. The value of these materials lies in the simplification and low cost of alloying carried out with their help, as well as their use as deoxidation agents (oxygen removal) in metal smelting.
Modern metallurgy has three methods for producing ferroalloys:
- Aluminothermic.
- Silicothermic.
- Carbon-reducing.
They are realized using smelting furnaces or electric furnaces.
Types of iron ores and their characteristics
From an economic point of view, they are classified primarily by their iron content:
- High – more than 55%. These are not natural formations, but already an industrial semi-finished product.
- Average. An example is sinter ore. Obtained from iron-rich natural raw materials through mechanical action.
- Low – less than 20%. These are obtained as a result of magnetic separation.
The location of ore mining is also economically important:
- Linear - lie in places of depressions in the earth's surface, the richest in iron, with a low content of sulfur and phosphorus.
- Flat-like - in nature they form on the surface of iron-containing quartzites.
According to geological parameters, in addition to hematites, the following are widespread and actively used:
- Brown iron ore (nFe2O3 + nH2O) is a metal oxide with the participation of water, usually based on limonites. Characteristic dirty yellowish color, loose, porous. Valuable metal contains from a quarter to fifty percent. Not much - but the substance is well restored. It is enriched for further production of good cast iron.
- Magnetic iron ore, magnetite is a natural iron oxide (Fe3O4). Hematite species are less common, but they contain more than 70% iron. They are dense and granular, in the form of crystals embedded in the rock, black and blue in color. Initially, the compound has magnetic properties; exposure to high temperatures neutralizes them.
- Spar iron ore containing siderite FeCO3.
- There is a large proportion of clay in the ore, then it is clay iron ore. A rare species with relatively low iron content and voids.
Deposits in Russia and the world
The Russian Federation has significant deposits of iron ore. The largest deposits in our country are:
- Kursk magnetic anomaly. According to experts, this is a quarter of the world's iron ore reserves, estimated at 200 billion tons, of which 30 billion are already highly enriched.
- The Bakcharskoye deposit in the Tomsk region is the second largest in Russia, containing vanadium and cobalt in its ore.
- There are also significant reserves of iron ore in the form of red iron ore in the Murmansk region. These are the Olenigorskoye and Kovdorskoye fields.
- The Kerch Peninsula of Crimea is rich in brown iron ores, which require further development.
- Significant iron deposits in Siberia are located in the area of the city of Kemerovo and in Altai.
- The Far Eastern region has deposits in the Amur region, the Republic of Sakha (Yakutia) and in the Khabarovsk Territory.
The largest foreign deposits include:
- Kirunavara - Sweden.
- Region of Lorraine - France.
- Krivoy Rog basin in Ukraine.
- Lower Saxony in Germany.
- Newfoundland and Labrador fields in Canada.
- Coastal region of Lake Superior in the USA.
- Port of Mayari - Cuba.
- El Pao and Cerro Bolivar - Venezuela.
- Itabira, Itabirita, Carajas in Brazil.
- The city of Conakry (Guinea) is home to the largest iron ore deposits in Africa.
- The so-called “iron belt” of India. States: Bihar and Orissa.
- Iron Monark, Iron Knob, Mount Goldsworthy, Mount Tom Price, Mount Nearman, Cockatoo and Hamersley in Australia.
World iron ore production: characteristics
There are 98 countries in the world that have extensive mineral deposits with high iron content. They are excellent for the industrial production of raw materials and their export to foreign countries. Russia has the largest reserves of iron ore, which is not surprising, given the geological structure and size of the territory.
Not only is a large amount of minerals concentrated here, but its quality characteristics are very different from its competitors. The largest iron ore region, the Kursk Magnetic Anomaly, is considered a unique deposit, and in terms of raw material reserves it has no analogues in the world. Despite this, the main exporter of metals to the world market is China.
A large number of deposits are located in Ukraine, Australia, and Brazil. Interestingly, China's reserves are much smaller than Russia's, but its production level is higher.
It is estimated that the planet contains up to 780 billion tons of iron ore, but only 477 billion tons have been explored so far. This is a huge amount of raw materials, which can provide humanity with valuable metal resources for a long time. It is also necessary to take into account the fact that the needs of metallurgy are slightly overlapped with the help of secondary raw materials.
Iron ore reserves by country.
| A country | Reserves (billion tons) | Production per year (million tons) | By iron content % |
| China | 9 | 250 | 9 |
| Brazil | 7,6 | 185 | 18 |
| Australia | 18 | 140 | 14 |
| Russia | 22,29 | 87 | 19 |
| USA | 10 | 60 | 6,4 |
| India | 1,8 | 75 | 4 |
| Ukraine | 16,84 | 75 | 11 |
| Canada | 12 | 35 | 7 |
In Ukraine, the Krivoy Rog field takes the lead in terms of reserves. It is characterized by hematites and magnetites with a high iron content (55-65%), which are high quality raw materials.
In the United States, 80% of resources are mined in the iron ore basin near Lake Superior. Most deposits are magnetite, with Fe content up to 55%. A huge amount of extracted raw materials is exported to the world market from here. In Canada, the main deposits are located on the island of Newfoundland and the province of Quebec. Both magnetites and hematites are mined there, the content of valuable raw materials in which reaches 57%.
Brazil is considered the country where the highest quality iron ore is mined in the world. The percentage of iron in minerals reaches 70%, which is rare in nature. The Itabiri and Itabirita deposits are filled with so-called lump ore, which is highly valued in the world.
The Australian raw material base is represented by hematites and lemonites (swamp ore). This is a type of mineral containing a large amount of iron, but its quality is inferior to magnetite. Most of the deposits are located on the western coast of the country, and the largest is Brocken. Indian deposits are rich not only in hematites, but also in alumina, so this mineral contains a high aluminum content, which negatively affects the quality.
87% of the world's ore production comes from low-quality raw materials. The iron content in such ores does not exceed 40%, and they require beneficiation.
Iron ore producing countries
- The current state of affairs in the global economy is determined by the strongest business activity of China, which occupies the top line of leaders in global iron ore production. It extracts 900 million tons per year annually.
- Australia is in second place - 420 million tons per year.
- Brazil produces 350 million tons of ore every year.
- India is in fourth place, supplying 245 million tons of iron to the market in 2019.
- Russia, which has the largest reserves, producing 100 million tons per year, holds fifth place. Which, as usual, speaks of our enormous unrealized potential.
Author: Yuri Florinskikh All articles by this author
Latest articles by the author: The largest producers of milk and dairy products in the world Diamonds: properties, mining methods and applications
How is iron ore mined?
Iron ore layers lie at different depths, which determines how it is extracted from the subsoil.
Quarry method of iron ore mining
Career way
The most common method of quarrying is used when deposits are found at a depth of about 200-300 meters. Development occurs through the use of powerful excavators and rock crushing plants. After which it is loaded for transportation to processing plants.
Extraction of iron ore in a mine
Mine method
The mine method is used for deeper layers (600-900 meters). Initially, a mine alignment is pierced, from which drifts are developed along the layers. From where the crushed rock is supplied “to the mountain” using conveyors. Ore from the mines is also sent to processing plants.
Borehole hydromining of iron
Borehole hydraulic production
First of all, for borehole hydraulic mining, a well is drilled to the rock layer. After that, pipes are brought into the target, and the ore is crushed with powerful water pressure for further extraction. But this method today has very low efficiency and is used quite rarely. For example, 3% of raw materials are extracted using this method, and 70% using the mine method.
After extraction, iron ore material must be processed to obtain the main raw material for metal smelting.
Kovdorskoye field
Due to its geological history, the Kola Peninsula has significant mineral deposits and makes a significant contribution to the Russian economy. The main iron ore deposits in this region began to be developed in 1962, although they were discovered before the war. The Kovdor iron ore deposit is one of the largest repositories of collectible raw materials in the state. Here are rare unique minerals that are not found anywhere else.
The Kovdor deposits have been developed since 1962; their reserves amount to about 650 million tons of magnetite ores. The width of the ore body is 100-800 meters, and the length stretches for more than a kilometer. Storeroom deposits have been explored to a depth of 800 meters. The average iron content is 28-30%. In addition to magnetite concentrate, baddeleyite and apatite concentrates are extracted from the ore.
Iron ore deposits
Iron ore deposits are usually divided according to their origin. In total, in geology it is customary to distinguish the following types:
- Magmatogenous, formed as a result of high temperature influences.
- Exogenous, originating in river valleys. Their formation was influenced by sedimentary processes and rock weathering.
- Metamorphogenic, which were formed in sedimentary deposits under the influence of various transformation processes, high temperatures and pressure.
Today, over 50 countries are involved in the iron mining industry and Russia is among the top five. In terms of the quantity of reserves, it ranks first and is only slightly inferior in terms of the quality of the iron itself.
Kursk magnetic anomaly
It is in it that more than half of the total supply of iron on the planet is found. The Kursk Magnetic Anomaly (KMA) is the world's largest iron ore basin. Most of it is located mainly in the following areas:
- Kursk;
- Orlovskaya;
- Belgorodskaya.
It is worth noting that in total its borders affect nine regions of central and southern Russia. Active development is underway at the following KMA fields:
- Stoilensky;
- Mikhailovsky;
- Lebedinsky.
KMA reserves amount to billions of tons, making it the world's largest deposit. However, the explored volumes are only 30 billion tons. Its area exceeds 160 thousand km². The ore mined there is represented by magnetite quartzites and granitoids.
The texture of the ore mined in KMA is multicomponent, and its depth varies from 30 to 650 meters. In the future, there will also be an opening for development of new deposits.
The Republic of Khakassia
Khakassia is home to some of the oldest iron ore deposits in Russia. Its base is represented by the Teysko-Balyksinsky, Abakan-Anzassky and Verkhneabakansky districts.
Abagas ore deposits in the Kuznetsk Alatau area and the Minusinsk Basin were discovered in 1933, but their development began only 50 years later. The dominant mineral here is magnetite, secondary roles are given to pyrite, hematite and musketovite. Balance reserves of raw materials amount to more than 73 million tons.
The Abakan iron ore deposit is located near the city of Abaza. Its deposits are represented by easily enriched skarn-magnetite ores. Balance reserves contain 145 million tons of ore, the average volume of iron is 42-45%. The deposit has been explored to a depth of 1300 meters.
Nature of origin
Most of the known mine types were formed under the influence of three main factors. The features and characteristics of iron ore actually depend on them.
Igneous formation. Igneous compositions were formed under the influence of high temperatures of magma or under conditions of high activity of ancient volcanoes. In fact, natural processes of mixing and melting of rocks took place.
This type of mineral is a crystalline mineral compound characterized by a high percentage of iron content. Deposits of igneous fossils can usually be found in ancient formation zones in mountainous areas. It was in these places that the molten substances came as close as possible to the surface layers of the soil.
Metamorphic formation. In the process of this formation, sedimentary type minerals are formed. The essence of this process comes down to the movement of individual sections of the Earth's crust, during which certain layers, rich in certain elements, fall under the rocks that lie above.
Minerals that were formed during the next movement migrate closer to the earth's surface. Iron ore, which is formed during metamorphic formation, typically has a high percentage of useful metallic compounds and is not located too deep from the surface. One of the most common examples is magnetic iron ore, which contains up to 75% iron.
Olenegorskoye field
The largest iron ore deposits in Russia include the Olenegorskoye deposit in the Murmansk region, which was discovered in 1932. Most of its raw material base is represented by ferruginous quartzites, the main minerals of which are magnetite and hematite. The presence of iron averages 31%. The ore lies almost to the surface, but the ore body goes to a depth of more than 800 meters with a length of 32 km. The ores of this deposit are easy to process; they have a minimal content of harmful impurities, which makes it possible to obtain high-quality metal.
According to the latest estimates, the reserves of the Olenegorsk deposit on the Kola Peninsula amount to 700 million tons of iron ore. The presence of such significant reserves is contained in very deep horizons, which creates the need for additional subsoil exploration.