Although a person, using the example of individual cutlery, has repeatedly been convinced that metal objects can turn out to be not heavy at all, nevertheless, metal appears to the latter primarily as something that is difficult to destroy under the influence of external forces, and therefore impressive in weight.
However, in this article we will talk about the lightest metals in the world: what properties they have, what they are used for and why they are of interest - the editors of 24SMI will tell you about this.
Titanium (Ti)
Discovered at the end of the 18th century and immediately added to the periodic table under number 22, a silver-colored chemical element with an atomic mass of 47.867 a. e.m. (atomic mass unit) and a density of 4.5 g/cm^3 is characterized by impressive strength.
Also among the properties of the metal, which has received the status of the hardest used, is excellent anti-corrosion resistance. This is also true for alloys produced on the basis of titanium, and the latter retain their own strength characteristics even at temperatures of 300 °C, which makes them indispensable in the current period of time in aviation and rocket science.
Titan, photo https://mining-prom.ru/
Named after the titans from ancient Greek mythology, the metal is one of the ten most common elements in nature, deposits of which have been discovered on all continents, excluding Antarctica. Moreover, Russia ranks second in the world in terms of concentration of ores containing the element in question after China.
In addition to the industries already mentioned, titanium alloys are in demand in shipbuilding, the chemical, automotive and defense industries, as well as in food production and agriculture. Due to its own inertness, titanium can easily contact the tissues of living organisms without causing chemical reactions hazardous to health, and therefore is actively used in medicine, from prosthetics and the manufacture of implants to the creation of surgical instruments.
Biological significance
The element was found among the permanent components in living organisms. In plants, it increases resistance to various diseases. The substance enhances the photochemical activity of chloroplasts in tomato foliage and the synthesis of nicotine in tobacco.
In the human body, lithium is produced primarily in the kidneys, but is also found in the thyroid gland, liver, heart, lungs and intestines. This element is involved in important processes of the human body:
- normalizes fat and carbohydrate metabolism;
- strengthens the immune system;
- prevents allergic reactions from developing;
- reduces nervous excitability.
In large quantities, the substance reduces the level of serotonin in the brain. If the sodium content in the body is high, lithium drugs are prescribed with caution, since the drugs can be hazardous to health and worsen kidney condition.
Aluminum (Al)
Aluminum is one of the most common non-ferrous metals. Discovered in 1825 and before the development of industrial production technology, it was more expensive than gold, an element with atomic number 13 and a mass of 26.982 a. e.m. has a density of 2.7 g/cm^3 and is distinguished by the presence of paramagnetic properties, although weak.
It conducts heat and electricity well, is not susceptible to corrosion, but is susceptible to mechanical damage, including easy bending. Alloys based on this light metal are characterized by ductility, satisfactory strength and are not susceptible to corrosion, and are also well welded.
Aluminum, photo: https://ru.wikipedia.org/
In terms of prevalence in the world, aluminum ranks first among metals and third among chemical elements of the periodic table, second only to oxygen and silicon. It is mined in more than 15 countries, the leaders among which are China, Russia and Canada. World reserves of this element are many times greater than the current need for its use.
The scope of use of aluminum and alloys based on this material is extensive. This includes ferrous metallurgy and pyrotechnics; it was even used for the manufacture of jewelry at a time when it was of exceptional value due to the undeveloped technical process. In Japan, it is still used in this capacity, sometimes replacing silver in jewelry.
Everyone knows about dishes and cutlery made from this flexible metal, but aluminum alloys that have the required characteristics in terms of strength are used as structural materials. Aluminum is also added to “automatic steels” to facilitate processing - thanks to it, a clear detachment of the part from the rod is achieved after processing is completed.
Industrial production
To obtain lithium by industrial methods, consumable raw materials are first prepared - minerals or salt solutions, which are extracted from salt lakes. Regardless of the method of extraction of consumable raw materials, the output is Li2CO3, which will undergo industrial processing.
Methods for obtaining consumable raw materials:
- electrolysis;
- recovery;
- refining.
The choice of an industrial method for producing alkali metal depends on the availability of equipment, the required result, and the type of consumable raw materials.
Beryllium (Be)
Unlike the previous ones, this metal, located in the table of chemical elements at number 4, is distinguished by a grayish color, as well as increased toxicity. It is characterized by fragility with comparative hardness exceeding that of aluminum and magnesium. Density - 1.8 g/cm^3. Atomic mass - 9 carbon units.
Discovered at the end of the 18th century, it was first obtained in its pure form only 30 years later, in 1828. It inherited its name from the mineral beryl, which, in turn, owes its name to the Indian city of Belur, famous for its deposit of emeralds - precious stones that are a variety of the mentioned rock.
Beryllium, photo: https://ru.m.wikipedia.org/
Beryllium is often found in dark-colored minerals, as well as in igneous rocks. Deposits containing this metal are located in South America and Africa. Production is also carried out on the Eurasian continent, mainly in India, Kazakhstan and Russia, within the boundaries of which there are two deposits - in the Sverdlovsk region and in Buryatia.
The metal is used in alloying alloys as an additive that makes the resulting materials harder, stronger and more resistant to corrosion. Beryllium's weak absorption of X-rays allows it to be used in the creation of gamma ray detectors. It is also used in nuclear energy as a neutron moderator. Beryllium alloys are used in aerospace engineering and for the manufacture of laser emitters.
The metal also conducts sound waves well, which is why it is used in the design of acoustic devices; however, due to the high complexity of processing to eliminate negative qualities, including toxicity, components made on its basis are characterized by increased cost. It is dangerous to humans - accumulating in the body, it leads to severe damage to the respiratory system, and is also characterized by a pronounced carcinogenic effect.
Properties
The properties of the element have been known to scientists for a long time. Compared to other alkali metals, it has a number of unique features, which determine the main areas of application of this substance.
Basic parameters of lithium according to the periodic table
Atomic number Z 3 Atomic mass 6.941 Group 1 Period 2 Belonging to the group alkali metals
Chemical
Properties:
- molar mass - 6.941;
- valency - 1;
- electronegativity - 1;
- atomic number - 3;
- covalent radius - 1.23 A;
- heat capacity - 3.307 kJ/(kg °C).
Lithium is stable when exposed to air. Of the group of alkali metals, it is the least active. It practically does not react when interacting with dry air and oxygen (provided that it is kept at room temperature).
The interaction of lithium with water is relatively calm. Upon contact with water, it begins to form an alkali and release oxygen. The metal floats on the surface of the liquid, quickly dissolving and emitting a characteristic hissing sound.
When the air is humid, the metal reacts with the gases it contains (especially nitrogen). An oxide film coats lithium surfaces when heated to 100–300°C. The film protects the metal from oxidative processes.
When reacting with sulfur, sulfide is formed (if heated to 130°C). It reacts with silicon when heated to 700°C. Dissolves in liquid ammonia, forming a blue solution.
Lithium cannot be stored in kerosene liquid. Due to its low density, the material will float to the surface. Mineral oil, gasoline, and paraffin are suitable for storage. It is better to choose a container made of tin. It must be hermetically sealed.
Lithium electronic circuit
Li:
1s2 2s1 →
Li-
: 1s22s2
The lithium ion -1 and Be have the same electronic configuration, +1B, +2C, +3N
The order of filling the shells of a lithium atom (Li-) with electrons: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
The 's' sublevel can have up to 2 electrons, the 's' level can have up to 6 electrons, the 'd' level can have up to 10 electrons, and the 'f' level can have up to 14 electrons.
Lithium has 3 electrons, let's fill the electron shells in the order described above:
2 electrons in the 1s sublevel
2 electrons in the 2s sublevel
Lithium oxidation state
Lithium atoms in compounds have oxidation states of 1, -1.
The oxidation state is the conditional charge of an atom in a compound: the bond in a molecule between atoms is based on the sharing of electrons, thus, if an atom’s charge virtually increases, then the oxidation state is negative (electrons carry a negative charge), if the charge decreases, then the oxidation state is positive.
Oxidation state of Li- ion = -1
Lithium ions
1+Li
Li 1-0Li
Valency of Li
Lithium atoms in compounds exhibit valency I.
The valency of lithium characterizes the ability of the Li atom to form chemical bonds. Valence follows from the structure of the electron shell of the atom; electrons involved in the formation of chemical compounds are called valence electrons. A more extensive definition of valency is:
The number of chemical bonds by which a given atom is connected to other atoms
Valence has no sign.
Quantum numbers Li 1-
Quantum numbers are determined by the last electron in the configuration; for the Li ion these numbers have the value N = 2, L = 0, Ml = 0, Ms = ½
Qualitative reactions
Qualitative reaction to alkali metals - coloring of the flame with salts of alkali metals .
Flame color: Li - carmine red Na - yellow K - violet Rb - brown-red Cs - violet-red
Physical properties of lithium:
| 400 | Physical properties | |
| 401 | Density | 0.534 g/cm3 (at 20 °C and other standard conditions, state of matter – solid), 0.512 g/cm3 (at a melting point of 180.50 °C and other standard conditions, the state of the substance is liquid), 0.507 g/cm3 (at 200 °C and other standard conditions, state of matter – liquid), 0.49 g/cm3 (at 400 °C and other standard conditions, state of matter – liquid), 0.474 g/cm3 (at 600 °C and other standard conditions, state of matter – liquid), 0.457 g/cm3 (at 800 °C and other standard conditions, state of matter – liquid), 0.441 g/cm3 (at 1000 °C and other standard conditions, state of matter – liquid) |
| 402 | Melting temperature* | 180.50 °C (453.65 K, 356.90 °F) |
| 403 | Boiling temperature* | 1330 °C (1603 K, 2426 °F) |
| 404 | Sublimation temperature | |
| 405 | Decomposition temperature | |
| 406 | Self-ignition temperature of a gas-air mixture | |
| 407 | Specific heat of fusion (enthalpy of fusion ΔHpl)* | 3.00 kJ/mol |
| 408 | Specific heat of evaporation (enthalpy of boiling ΔHboiling)* | 136 kJ/mol |
| 409 | Specific heat capacity at constant pressure | 3.4122 J/g K (at 25°C) |
| 410 | Molar heat capacity | 24.86 J/(K mol) |
| 411 | Molar volume | 13.1 cm³/mol |
| 412 | Thermal conductivity | 84.8 W/(mK) (at standard conditions), 84.8 W/(mK) (at 300 K) |
| 413 | Thermal expansion coefficient | 46 µm/(MK) |
| 414 | Thermal diffusivity coefficient | |
| 415 | Critical temperature | |
| 416 | Critical pressure | |
| 417 | Critical Density | |
| 418 | Triple point | |
| 419 | Vapor pressure (mmHg) | |
| 420 | Vapor pressure (Pa) | |
| 421 | Standard enthalpy of formation ΔH | |
| 422 | Standard Gibbs energy of formation ΔG | |
| 423 | Standard entropy of matter S | |
| 424 | Standard molar heat capacity Cp | |
| 425 | Enthalpy of dissociation ΔHdiss | |
| 426 | The dielectric constant | |
| 427 | Magnetic type | |
| 428 | Curie point | |
| 429 | Volume magnetic susceptibility | |
| 430 | Specific magnetic susceptibility | |
| 431 | Molar magnetic susceptibility | |
| 432 | Electric type | |
| 433 | Electrical conductivity in the solid phase | |
| 434 | Electrical resistivity | |
| 435 | Superconductivity at temperature | |
| 436 | Critical magnetic field of superconductivity destruction | |
| 437 | Prohibited area | |
| 438 | Charge carrier concentration | |
| 439 | Mohs hardness | |
| 440 | Brinell hardness | |
| 441 | Vickers hardness | |
| 442 | Sound speed | |
| 443 | Surface tension | |
| 444 | Dynamic viscosity of gases and liquids | |
| 445 | Explosive concentrations of gas-air mixture, % volume | |
| 446 | Explosive concentrations of a mixture of gas and oxygen, % volume | |
| 446 | Ultimate tensile strength | |
| 447 | Yield strength | |
| 448 | Elongation limit | |
| 449 | Young's modulus | |
| 450 | Shear modulus | |
| 451 | Bulk modulus of elasticity | |
| 452 | Poisson's ratio | |
| 453 | Refractive index |
Lithium crystal lattice:
| 500 | Crystal cell | |
| 511 | Crystal grid #1 | |
| 512 | Lattice structure | Cubic body-centered |
| 513 | Lattice parameters | 3.510 Å |
| 514 | c/a ratio | |
| 515 | Debye temperature | 400 K |
| 516 | Name of space symmetry group | Im_ 3m |
| 517 | Symmetry space group number | 229 |
| 521 | Crystal grid #2 | |
| 522 | Lattice structure | Hexagonal close-packed |
| 523 | Lattice parameters | a = 3.111 Å, c = 5.093 Å |
| 524 | c/a ratio | 1,637 |
| 525 | Debye temperature | |
| 526 | Name of space symmetry group | P63/mmc |
| 527 | Symmetry space group number | 194 |
Electrical properties of lithium
Type of electrical conductivity conductor
Magnetic properties of lithium
Type of magnetic permeability paramagnetic
Thermodynamic properties of lithium
Physical state under normal conditions solid Melting point Kelvin 453.69 (Kelvin) Melting point Celsius 180.54 (°C) Boiling point Kelvin 1615.15 (Kelvin) Boiling point Celsius 1342 (°C)
Optical properties of lithium
Color Silver Emission spectrum of lithium Absorption spectrum of lithium
Mechanical properties of lithium
Density of solids 0.535 103 (Kilogram/Meter 3) Speed of sound 6000 (Meter/Second)
Magnesium (Mg)
Located at number 12 in the periodic table, it is a malleable metal with an atomic mass of 24.307 a. e.m. and a density of 1.7 g/cm^3 was first obtained in its pure form in 1808. Plastic and easy to press and cut.
Characterized by a high melting point (650 °C) and corrosion resistance. When creating alloys based on magnesium, the mechanical characteristics of the metal increase significantly, which greatly expands the scope of application of this type of material.
Magnesium, photo: https://infonew.do.am/
The element is included in the list of the most common on Earth and is found both in the crust and in sea water, usually in the composition of salts and minerals. Natural deposits of native magnesium are extremely rare - a couple of them are located in Russia, Eastern Siberia, and Tajikistan. The United States is considered the leader in magnesium production in 2020.
It is mainly used to produce all kinds of alloys, both light and ultra-light, the scope of which is aircraft and automotive manufacturing. Also, due to its flammable properties, it is used in pyrotechnics and in the creation of incendiary and illumination rockets in the defense industry.
Without magnesium powder with added oxidizing agents, photography would previously have been impossible - although magnesium flashes are used much less frequently than in the past, there is still a demand for them. Magnesium is also a substance important for the normal functioning of the body and the course of metabolic processes, so drugs based on it are used in medicine - in cardiology, neurology and in the fight against gastroenterological disorders.
Lithium (Li)
So it comes to the element, which is the lightest metal in the world. The density of lithium, located at number 3 in the periodic table, is only 0.5 g/cm^3, which is less than that of water, so pure lithium does not sink. The atomic mass of the element ranges from 6.398 to 6.997 a. e.m. depending on the isotope. It was discovered in 1817, and obtained in metal form just a year later.
It is characterized by increased chemical activity and therefore easily forms complex compounds in nature. Plastic, easy to process by rolling and pressing. Color: silver. At room temperature it reacts weakly with oxygen. Ignition occurs at 300 °C.
Lithium, photo: https://ru.m.wikipedia.org/
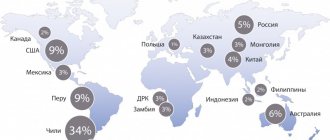
It occurs naturally in rock-forming minerals and in highly saline lake sediments. Among the developed deposits, the most famous are Chilean, Australian and Argentinean, although they are also found in other countries, including China. In Russia, the main accumulation of rocks containing lithium is in the Murmansk region. Since 2021, an installation for the extraction of metal from ores with low element content has been operating in the country in an experimental format, thanks to which the procedure is possible with insignificant financial and labor costs.
Lithium salts are used in the creation of laser equipment and optics, as oxidizing and reducing agents in the chemical industry, as well as in medicine and various industries, including textiles (as bleaches), food (as preservatives) and cosmetics. Lithium alloys are used to make high-performance conductors, including anodes needed for electrolysis.
The element is also used in the creation of batteries, including alkaline ones, and not just solid-state ones. In small quantities, lithium is required by the human body, since it is involved in metabolism and also affects psycho-emotional excitability and immune defense.
History of discovery and study
The first sample of lithium metal was obtained thanks to the work of Humphry Davy. Using an electric current, he decomposed the molten hydroxide of this alkali metal. After some time, Leopold Gmelin experimented with lithium-containing salts. He was able to detect that they paint the flames a dark color.
The main credit for the discovery of a new chemical element and the growth of its popularity belongs to Johann August. In 1817, he discovered a new substance in petalite, an aluminosilicate. After some time, lithium was found in other mineral formations. It received this name due to the fact that it was first found in stones. The name of the stone in Greek is “lithos”.
Lithium prevalence
The Universe consists of 6×10-7% lithium The sun consists of 6×10-9% lithium The world's oceans consist of 0.000018% lithium The human body consists of 3×10-6% lithium