Titanium is considered one of the most durable metals. It perfectly withstands both mechanical loads and use in aggressive environmental conditions. But under certain conditions, titanium also begins to deteriorate. If you do not react to a problem in time, you may face complete destruction of the material.
This article will talk about how titanium and its alloys behave when faced with external catalysts for corrosion development. An equally important topic will also be touched upon - methods of prevention and additional means to protect the material from negative external influences and gradual destruction in various environments.
Features of the corrosion process
The basis of the corrosion process is oxidation. It is provoked by external factors - humidity, contact with acids, alkalis and other potential threats.
Titanium belongs to the category of metals that resist negative influences well. But when creating incorrect conditions and accumulating a sum of factors, a real problem arises associated with the gradual destruction of the material.
When corrosion develops, there is a great danger that the material will become completely unusable. It loses its strength and begins to collapse. Without the right approach to protecting and limiting the impact of negative external conditions, you can quickly lose even the most expensive products.
The catalyst for the process is contact with an oxidizing agent. Therefore, below we will tell you how the alloy behaves in different types of aggressive environments.
Application
In pure form and in the form of alloys
Titanium Alloy Watch
Blank of a titanium frame for an F-15 fighter before and after pressing on an Alcoa 45 thousand ton stamping press, May 1985
The use of titanium metal in many industries is due to the fact that its strength is approximately equal to the strength of steel, although it is 45% lighter . Titanium is 60% heavier than aluminum, but is about twice as strong.
- Titanium in the form of alloys is the most important structural material in aircraft, rocket and shipbuilding.
- The metal is used in the chemical industry (reactors, pipelines, pumps, pipeline fittings), military industry (body armor, aviation armor and fire barriers, submarine hulls), industrial processes (desalination plants, pulp and paper processes), automotive industry, agricultural industry , food industry, sporting goods, jewelry, mobile phones, light alloys, etc.
- Titanium is physiologically inert, which is why it is used in medicine (prostheses, osteoprostheses, dental implants), in dental and endodontic instruments, and piercing jewelry.
- Titanium casting is performed in vacuum furnaces into graphite molds. Vacuum lost wax casting is also used. Due to technological difficulties, it is used in artistic casting to a limited extent. The first monumental cast titanium sculpture in the world is the monument to Yuri Gagarin on the square named after him in Moscow.
- Titanium is an alloying additive in many alloy steels and most special alloys.
- Nitinol (nickel-titanium) is a shape memory alloy used in medicine and technology.
- Titanium aluminides are very resistant to oxidation and heat-resistant, which, in turn, determined their use in aviation and automotive manufacturing as structural materials.
- Titanium is one of the most common getter materials used in high-vacuum pumps.
There are many titanium alloys with different metals. Alloying elements are divided into three groups, depending on their effect on the temperature of the polymorphic transformation: beta stabilizers, alpha stabilizers and neutral strengtheners. The first ones lower the transformation temperature, the second ones increase it, the third ones do not affect it, but lead to solution strengthening of the matrix. Examples of alpha stabilizers: aluminum, oxygen, carbon, nitrogen. Beta stabilizers: molybdenum, vanadium, iron, chromium, nickel. Neutral hardeners: zirconium, tin, silicon. Beta stabilizers, in turn, are divided into beta isomorphic and beta eutectoid-forming.
The most common titanium alloy is the Ti-6Al-4V alloy (VT6 in the Russian classification), containing about 6% aluminum and about 4% vanadium. Based on the ratio of crystalline phases, it is classified as an (α+β) alloy. Up to 50% of mined titanium is used for its production.
Ferrotitanium (an alloy of titanium with iron containing 18-25% titanium) is used in ferrous metallurgy to deoxidize steel and remove unwanted impurities dissolved in it (sulfur, nitrogen, oxygen).
In the 1980s, about 60-65% of titanium mined in the world was used in the construction of aircraft and rockets, 15% in chemical engineering, 10% in energy, 8% in shipbuilding and for water desalination plants.
In the form of connections
- White titanium dioxide (TiO2) is used in paints (such as titanium white) and in the production of paper and plastics. Food additive E171.
- Organo-titanium compounds (for example, tetrabutoxytitanium) are used as a catalyst and hardener in the chemical and paint and varnish industries.
- Inorganic titanium compounds are used in the chemical electronics and fiberglass industries as additives or coatings.
- Titanium carbide, titanium diboride, and titanium carbonitride are important components of superhard materials for metal processing.
- Titanium nitride is used to coat instruments, church domes and in the production of costume jewelry, as it has a color similar to gold.
- Barium titanate BaTiO3, lead titanate PbTiO3 and a number of other titanates are ferroelectrics.
- Titanium tetrachloride is used to iridize glass and to create smoke screens.
Analysis of consumption markets
In 2005, Titanium Corporation
published the following estimate of titanium consumption in the world:
- 60% - paint;
- 20% - plastic;
- 13% - paper;
- 7% - mechanical engineering.
Features of the interaction of titanium with various types of aggressive environments
As noted above, titanium belongs to the list of materials that have good natural protection against the development of the corrosion process. In many environments, high temperatures must be maintained for corrosion to occur. At the same time, the metal itself practically does not enter into chemical reactions with various types of substances.
The main protective factor is the formation of a thin film on the surface of titanium. It does not allow contact with the external environment and acts as a barrier to oxidizing agents. An interesting feature of titanium that distinguishes it from other types of materials is that even when such a film is removed, it appears again due to the passivation process. Thus, the metal has the property of self-protection from destructive effects.
The behavior of titanium itself will change depending on what conditions were created around the part. Let's look at the most common ones.
Nitric acid
Nitric acid is one of the list of strong factors that provoke the development of the oxidative process. When different types of metals are placed in such an environment, dissolution may occur at different rates. But titanium belongs to the category of products that are not affected by nitric acid.
Regardless of the concentration of the solution, corrosion of titanium proceeds very slowly. In a year you can get a maximum value of no more than 0.2 mm.
The only thing that can threaten the metal is red fuming nitric acid. An intense reaction occurs in it, as a result of which corrosion rapidly develops. The only means to neutralize the process is to add a small amount of water.
Hydrochloric acid
Hydrochloric acid affects titanium much more intensely than nitric acid. Much depends on the temperature and concentration of the solution in which the material is used. Diluted solutions pose the least danger.
At room temperature, the intensity of corrosion gradually increases as the percentage of the main substance in the solution increases. A significant catalyst for speed is an increase in temperature. So even in very weak solutions when heated to 100 degrees, the corrosion rate becomes much higher. If the solution becomes more saturated, the intensity only becomes higher. Example - if you heat a 20% hydrochloric acid solution to a temperature of 60 degrees and immerse a titanium part in it, the corrosion intensity will increase to 29.8 mm per year - this is a very high rate of deterioration of the material, which can lead to its complete failure.
The passivating film on the metal surface becomes increasingly thin and is quickly removed. It is also worth remembering that even with the strong negative impact of hydrochloric acid, the risk of damage to titanium remains less than in the case of stainless steel under similar conditions.
Sulfuric acid
In solutions with low concentrations of titanium corrosion there is no need to worry. Even if a one percent sulfuric acid solution is heated to a temperature of 95 degrees, the level of damage will remain low.
More concentrated solutions behave similarly, up to 20%, if the ambient temperature does not rise above normal room temperature.
With increasing temperature, the corrosion process becomes more intense. So, if you heat up a 20% sulfuric acid solution very much, titanium may begin to gradually dissolve. The corrosion rate per year reaches 10 mm. There are proven methods to reduce the rate of dissolution. To do this, you need to add other types of acids to the composition - chromic, manganese, nitric or others.
Organic acids
The material performs well with most organic acids; virtually no chemical reaction is observed. Even if we are talking about tartaric, acetic and lactic acid, titanium remains intact and the protective film on its surface is intact.
Molten metals
When contacting molten metals, the type of titanium alloy is of great importance. So pure material, even in a highly heated molten environment, does not begin to rust when in contact with potassium, tin, magnesium, mercury and other potentially dangerous aggressive substances.
Hydrofluoric acid
This solution is the most dangerous for titanium. Even a weak, one percent solution greatly increases the rate of the corrosion process. With increasing concentration, titanium parts begin to melt quickly. And in this respect, the composition is largely similar in its behavior to other types of metals and alloys.
Other types of acids
The titanium part can also be placed in various types of acids. These include hydrofluorosilicic and phosphoric.
The material perfectly resists damage when in contact with alcohols, hydrogen peroxide, bromine, chlorine and many others.
In order to increase the corrosion resistance of titanium, additional oxidizing agents and inhibitors can be used. Both copper and iron in varying degrees of concentration can be used as such an inhibitor.
The materials can also be used with other metals, which significantly increase corrosion resistance. These include:
- Hafnium.
- Tantalum.
- Tungsten.
- Zirconium and much more.
Next, we will also talk about how alloying helps to greatly increase the quality of the material and significantly increase the duration of its use.
Metal oxidation
Oxidation is a special type of procedure for coating a metal material with an oxide film. As a result of this process, a thin film appears on the metal surface, which performs a barrier function. It protects the material from air and moisture.
Oxidation of metal is one of the most effective methods for protecting it from the formation of rust on the surface. The film covers it with a fairly dense layer. After the procedure, all metal oxidation processes completely stop. As a result, products that are processed using the oxidation method last longer and retain their attractive external qualities for many years.
This processing procedure for various types of products is used not only to protect metal products from corrosion. This function is known to many. However, in some situations it is used to give a metal product decorative qualities.
Today, many types of metals undergo oxidation.
In this regard, the following are highlighted:
Aluminum oxidation
This procedure occurs quite often. It is used for:
Anodic oxidation of aluminum
Chemical oxidation of aluminum
Electrochemical oxidation of aluminum
As a result, after processing, the metal receives a small layer of oxide film, which has excellent protective qualities.
The procedure itself does not take much time. It is carried out after preliminary preparation of the metal. Its surface must be clean and degreased so that the oxide film has better adhesion to the aluminum.
For aluminum, another technology called color oxidation of aluminum is used. Due to this, a film of a certain color is formed on the surface of the metal. This process is decorative in nature. The effect of this method lasts for quite a long period of time.
Steel oxidation
Today, oxidation of steel products is not uncommon. They are susceptible to the formation of a corrosive film.
Chemical oxidation of steel
To process steel material, a chemical type of oxidation is used. It consists in immersing steel in a specially prepared acidic solution, which promotes the formation of an oxide film on the surface of the steel. It has a small thickness. However, it has a high level of durability.
Before the metal is treated with an oxidizing agent, it is carefully prepared. To do this, special products are used to remove dirt and greasy film.
Titanium oxidation
As is known, metals such as titanium and its alloys have a low level of wear resistance. In order for the metal to acquire strength and hardness, various methods are used. One of them is oxidation. Thanks to it, a protective film appears on the surface of the metal, which increases the strength of titanium significantly.
Table 1. Metal oxidation - surface preparation.
| Composition and mode | Solution number | ||
| 1 | 2 | 3 | |
| Composition, mass fraction, % | |||
| sulfuric acid (density 1.8 g/cm3) | — | 90—92 | 20—30 |
| nitric acid (density 1.4 g/cm3) | 95-97 | 5-6 | 40—60 |
| hydrofluoric acid or its salts | 3-5 | 0,5—1 | 10—12 |
| Operating temperature, K | 290—300 | 290—300 | 290—300 |
| Shutter speed, min | 0,1—0,2 | 1—2 | 0,2—0,3 |
Alloying as a method of protecting titanium from corrosion
One of the most common and well-proven means of protecting titanium from corrosion is the use of additional alloying elements. All of them are divided into several groups. These include:
- First. These are elements with a low passivating effect. The addition of elements such as Mo, Ta, Nb shows itself best. The main advantage of using alloying of an element of the first group is a decrease in the activity of the anodic process. At the same time, the environment itself can also greatly influence how exactly the alloying element affects the stability of the metal.
- Second. The second group includes elements such as Cr, Ni, Mn, Fe. An important difference between the elements is that they have their own high protective corrosion properties. The materials provide best corrosion protection when used in acids with low oxidation rates.
- Third. There are several categories of elements - Al, Sn, O, N. The corrosion resistance of titanium is higher when alloyed, regardless of the state - both passive and active. Good parameters are also provided when introducing the material into neutral environments. The level of negative impact in this case is low, because the films on the surface of titanium do not change their composition.
- Fourth. The most effective elements are Cu, W, Mo, Ni, Re. It is best to use such an alloying agent in order to slow down or completely eliminate the cathodic process.
It is also worth paying attention to what material is used for alloying. It is best shown by the use of substances such as niobium and molybdenum. You can also actively use tantalum and zirconium.
Corrosion of titanium and titanium alloys
The creation of new technologies and production leads to the use of aggressive environments. The use of the latter raises the question of structural materials resistant to their effects. Of great interest in this regard are the metals of the subgroups titanium and vanadium. They have already found application in modern instrument making. For example, they are widely used in rocket and aircraft technology, as well as in the creation of nuclear reactors.Titanium and titanium alloys are widely used in various industries due to their high specific strength and corrosion resistance.
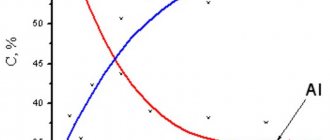
VT6 alloy is one of the first domestic structural titanium alloys. Table 1 shows the chemical composition of the VT6 alloy.
Table 1 - Chemical composition of titanium alloy VT6.
Basic
elements
| Al | V | Impurities | Fe | Si | O | C | N | H | Zr | |
| Content, % | 6,0 | 4,0 | Content no more than, % | 0,3 | 0,1 | 0,2 | 0,1 | 0,05 | 0,015 | 0,3 |
Titanium can participate in many compounds; it is chemically very active. And at the same time, titanium is one of the few metals with exceptionally high corrosion resistance: it is practically eternal in air, in cold and boiling water, and is very resistant in sea water, in solutions of many salts, inorganic and organic acids. In terms of its corrosion resistance in sea water, it surpasses all metals, with the exception of noble ones - gold, platinum, etc., most types of stainless steel, nickel, copper and other alloys. In water and in many aggressive environments, pure titanium is not subject to corrosion. Why is this happening? Why is titanium, which reacts with almost all elements of the periodic table, so actively, and often violently, with explosions, resistant to corrosion?
General understanding of metal corrosion
The production of metals from their natural compounds is always accompanied by a significant expenditure of energy. The only exceptions are metals that occur in nature in free form: gold, silver, platinum, mercury. The energy spent on producing metals accumulates in them as Gibbs free energy and makes them chemically active substances that, as a result of interaction with the environment, become positively charged ions:
Men++ ne ? Me0 (G>0); Ме0 – ne ? Me n+ (G
The spontaneous process of destruction of metals as a result of interaction with the environment, occurring with the release of energy and dispersion of matter (increase in entropy), is called corrosion. Corrosion processes proceed irreversibly in accordance with the second law of thermodynamics.
It is estimated that about 20% of the annual metal smelting is spent in corrosion processes. Corrosion causes great harm in mechanical engineering, since due to the corrosion destruction of any one part, a machine that often costs tens and hundreds of thousands of rubles can fail. Corrosion reduces the accuracy of instrument readings and the stability of their operation over time. Minor corrosion of the electrical contact will cause it to fail when turned on. Measures to combat corrosion processes are an urgent task of modern technology.
Corrosion processes are significantly influenced by the level of external or internal (residual) stresses and their distribution in the metal of the product.
Parts and components of machines operating at high temperatures are susceptible to chemical corrosion - piston and turbine engines, rocket engines, etc. The chemical affinity of most metals for oxygen at high temperatures is almost unlimited, since the oxides of all technically important metals can dissolve in metals and leave the equilibrium system:
2Me(s) + O2(g) 2MeO(s);
MeO(t) [MeO] (solution)
Under these conditions, oxidation is always possible, but along with the dissolution of the oxide, an oxide layer also appears on the surface of the metal, which can inhibit the oxidation process.
The rate of metal oxidation depends on the rate of the chemical reaction itself and the rate of diffusion of the oxidizing agent through the film, and therefore the protective effect of the film is higher, the better its continuity and the lower its diffusion ability. The continuity of the film formed on the surface of the metal can be assessed by the ratio of the volume of the formed oxide or some other compound to the volume of the metal spent on the formation of this oxide (Pilling-Badwords factor).
Coefficient ? (Pilling-Badwords factor) has different values for different metals and is given in Table 2.
Table 2. The value of the coefficient? for some metals
| Metal | Oxide | ? | Metal | Oxide | ? |
| Mg | MgO | 0.79 | Zn | ZnO | 1.58 |
| Pb | PbO | 1.15 | Zr | ZrO2 | 1.60 |
| Cd | CdO | 1.27 | Be | BeO | 1.67 |
| Al | Al2O2 | 1.31 | Cu | Cu2O | 1.67 |
| Sn | SnO2 | 1.33 | Cu | CuO | 1.74 |
| Ni | NiO | 1.52 | Ti | Ti2O3 | 1.76 |
| Nb | NbO | 1.57 | Cr | Cr2O3 | 2.02 |
| Nb | Nb2O3 | 2.81 |
Continuous and stable oxide layers are formed at? = 1.2—1.6, but at large values? the films are not continuous, easily separated from the metal surface (iron scale) as a result of internal stresses.
Behavior of titanium and its alloys in various aggressive environments
Reactions of titanium with many elements occur only at high temperatures. At ordinary temperatures, the chemical activity of titanium is extremely low and it practically does not react. This is due to the fact that on the fresh surface of pure titanium, as soon as it is formed, an inert, extremely thin (several angstrom (1A = 10-10m)) film of titanium dioxide very quickly appears, merging well with the metal, protecting it from further oxidation. Even if remove this film, then in any environment containing oxygen or other strong oxidizing agents (for example, in nitric or chromic acid), this film appears again, and the metal, as they say, is “passivated” by it, that is, it protects itself from further destruction.
Let us consider in more detail the behavior of pure titanium in various aggressive environments: such as nitric, hydrochloric, sulfuric, aqua regia and other acids and alkalis.
In nitric acid, which is a strong oxidizing agent in which many metals quickly dissolve, titanium is exceptionally resistant. At any concentration of nitric acid (from 10 to 99%), at any temperature, the corrosion rate of titanium does not exceed 0.1–0.2 mm/year. Only red fuming nitric acid, supersaturated (20% or more) with free nitrogen dioxides, is dangerous: pure titanium reacts violently and explosively in it. However, as soon as you add at least a little water (1–2% or more) to such an acid, the reaction ends and the corrosion of titanium stops.
Titanium is stable in hydrochloric acid only in dilute solutions. For example, in 0.5% hydrochloric acid, even when heated to 100° C, the corrosion rate of titanium does not exceed 0.01 mm/year, in 10% at room temperature the corrosion rate reaches 0.1 mm/year, and in 20% at 20° C – 0.58 mm/year. When heated, the corrosion rate of titanium in hydrochloric acid increases sharply. Thus, even in 1.5% hydrochloric acid at 100° C, the corrosion rate of titanium is 4.4 mm/year, and in 20% hydrochloric acid when heated to 60° C, it is already 29.8 mm/year. This is explained by the fact that hydrochloric acid, especially when heated, dissolves the passivating film of titanium dioxide and the metal begins to dissolve. However, the corrosion rate of titanium in hydrochloric acid under all conditions remains lower than that of stainless steels.
In low concentration sulfuric acid (up to 0.5–1%), titanium and most of its alloys are resistant even at solution temperatures up to 50–95° C. Titanium is also resistant in more concentrated solutions (10–20%) at room temperature, under these conditions, the corrosion rate of titanium does not exceed 0.005–0.01 mm/year. But with increasing temperature of the solution, titanium in sulfuric acid, even at a relatively weak concentration (10–20%), begins to dissolve, and the corrosion rate reaches 9–10 mm/year. Sulfuric acid, like hydrochloric acid, destroys the protective film of titanium dioxide and increases its solubility. It can be sharply reduced if a certain amount of nitric, chromic, manganese acids, chlorine compounds or other oxidizing agents are added to solutions of these acids, which quickly passivate the titanium surface with a protective film and stop its further dissolution. That is why titanium is practically the only metal that does not dissolve in “regia vodka”: in it, at ordinary temperatures (10–20 ° C), titanium corrosion does not exceed 0.005 mm/year. Titanium also slightly corrodes in boiling “regia vodka”, but in it, as is known, many metals, and even such as gold, dissolve almost instantly.
Titanium corrodes very weakly in most organic acids (acetic, lactic, tartaric), in dilute alkalis, in solutions of many chloride salts, and in physiological solution. But titanium interacts very violently with chloride melts at temperatures above 375° C.
In the melt of many metals, pure titanium exhibits amazing resistance. In hot liquid magnesium, tin, gallium, mercury, lithium, sodium, potassium, and in molten sulfur, titanium practically does not corrode, and only at very high melt temperatures (above 300–400 ° C) its corrosion rate in them can reach 1 mm/ year. However, there are many aggressive solutions and melts in which titanium dissolves very intensively.
The main “enemy” of titanium is hydrofluoric acid (HF). Even in a 1% solution, the corrosion rate of titanium is very high, and in more concentrated solutions, titanium “melts” like ice in hot water. Fluorine, this “destroying everything” (Greek) element, reacts violently with almost all metals and burns them.
Titanium cannot withstand even low concentrations of hydrofluorosilicic and phosphoric acids, hydrogen peroxide, dry chlorine and bromine, alcohols, including alcohol tincture of iodine, and molten zinc. However, the resistance of titanium can be increased by adding various oxidizing agents - so-called inhibitors, for example, to solutions of hydrochloric and sulfuric acids - nitric and chromic. Inhibitors can also be ions of various metals in solution: iron, copper, etc.
Some metals can be introduced into titanium, increasing its resistance tens and hundreds of times, for example, up to 10% zirconium, hafnium, tantalum, tungsten. The introduction of 20–30% molybdenum into titanium makes this alloy so resistant to any concentrations of hydrochloric, sulfuric and other acids that it can even replace gold in working with these acids. The greatest effect is achieved through the addition of four platinum group metals to titanium: platinum, palladium, rhodium and ruthenium. Just 0.2% of these metals is enough to reduce the corrosion rate of titanium in boiling concentrated hydrochloric and sulfuric acids by tens of times. It should be noted that noble platinoids only affect the durability of titanium, and if they are added to, say, iron, aluminum, magnesium, the destruction and corrosion of these structural metals does not decrease.
The influence of alloying elements in titanium on corrosion resistance
All alloying elements present in titanium can be divided into four groups based on corrosion resistance.
The first group includes easily passivated elements that increase the corrosion resistance of titanium by inhibiting the anodic process (to varying degrees and depending on the nature of the medium). This group includes the following most important alloying agents: Mo, Ta, Nb, Zr, V (arranged in descending order of beneficial effect on corrosion resistance).
The second group of metals that have a similar effect on the corrosion resistance of titanium includes Cr, Ni, Mn, Fe. These elements, some of which are themselves corrosion-resistant (Cr, Ni), although not greatly, reduce the corrosion resistance of titanium, especially in non-oxidizing acids as titanium alloying increases.
The third group of alloying elements that have a common effect on the corrosion resistance of titanium includes Al, Sn, O, N, C. It has been established that aluminum additives reduce the corrosion resistance of titanium in the active and passive states. In neutral environments, aluminum (up to 5% Al), although it has a negative effect, is small. The decrease in corrosion resistance when alloying with aluminum is associated with the facilitation of the anodic and cathodic processes due to changes in the chemical nature of passive films.
The fourth group of alloying elements that similarly affect the corrosion resistance of titanium includes metals with low resistance to the cathodic process. In order of increasing effectiveness of their impact on titanium, these elements are arranged in the following row: Cu, W, Mo, Ni, Re, Ru, Pd, Pt.
It has been proven that the introduction of elements such as molybdenum, niobium, zirconium, and tantalum into titanium alloys is not limited in quantity. They increase corrosion resistance and help increase strength.
Electrochemical corrosion under the influence of internal macro- and microgalvanic couples
Previously, electrochemical corrosion was called galvanic corrosion, since the destruction of the metal occurs under the influence of the resulting galvanic couples.
Let us consider various cases of the occurrence of corrosive galvanic couples.
1. Contact with an electrolyte of two different metals in the case of a combination in one assembly or part of metals of different activity in a given environment, or in the case of using a eutectic type alloy of two metals of different activity.
2. Contact between a metal and its compound having metallic or semiconductor properties. In any case, the free metal has a negative electrical charge, and the compound has a positive charge, since some of its conduction electrons are bound. This is also true for intermetallic compounds.
3. Various concentrations of electrolytes or air dissolved in the liquid electrolyte.
4. Different levels of mechanical stress in the same part.
Let us consider in more detail the last case of the occurrence of a corrosive galvanic couple. Corrosive vapors can arise under the action of external or internal mechanical stresses (residual stresses, for example during welding). If a plate of steel, duralumin or titanium alloy is bent and immersed in a stressed state in a corrosive environment, then cracks will appear in the stretched layer (outer) in a relatively short time (Fig. 1), and the inner compressed layer will remain unchanged. Tensile forces are especially dangerous, since in this case the metal increases its activity.
Figure 1 — Corrosion of a plate in a stressed state
If an elastically bent plate (see Fig. 1) is thermally treated and elastic deformations turn into plastic ones (relaxation phenomenon), then a potential difference does not arise. Thus, in the manufacture of parts and assemblies of machines to relieve residual stresses, the products should always be thermally treated if these products are intended to operate in highly correlated environments.
For this purpose, in the production of thin sheets of SMC - alloy VT6, obtained by isothermal rolling, at the Institute of Applied Mathematics and Mathematics of the Russian Academy of Sciences, creep annealing is used to more completely remove residual stresses and form a grain structure, which consists of the following: the sheets are stacked between flat strikers and pressed under pressure 3 -5 MPa at a temperature of 550? C. After 20 minutes of exposure, the heating is turned off, and the package cools down along with the stamping block for 12 hours.
Features of the interaction of titanium with air.
Air, which is a mixture of various gases, is a complex gas phase, the effect of which on titanium can be very diverse. In this case, the interaction of titanium with oxygen in the air differs from the interaction of titanium with pure oxygen, since this interaction is influenced by nitrogen and other components of the air. At the same time, it should be borne in mind that with all the complexity of the gas phase (air), its effect on titanium should be considered primarily as a reaction of interaction with it of the most active and quite significant component - oxygen.
Interaction of titanium with oxygen.
When titanium interacts with oxygen, various phases of chemical compounds and solid solutions are formed.
At sufficiently low temperatures, the interaction of titanium with oxygen is limited to adsorption. The initial heat of oxygen adsorption on titanium at 25°C is 989 kJ/mol; the initial adhesion coefficient is 1; 0.8 and 0.67 at temperatures -196; 25 and 300OS respectively. With further interaction, an oxide film is formed on the titanium surface.
In accordance with thermodynamic calculations, the oxide film on titanium should consist of layers of oxides in the sequence:
Ti6O®Ti3O®Ti2O®Ti3O2®TiO®Ti13O5®TiO2
In fact, during the oxidation of titanium at temperatures below 300°C, the oxide layers consist mainly of Ti3O5; during oxidation in the temperature range 400–800°C, predominantly rutile TiO2 is formed, and at temperatures above 800°C, oxides TiO and Ti2O3 are found. According to the work, the oxidation of titanium in air and oxygen to temperatures of Ј 600-650°C is accompanied by the formation of thin oxide films with a thickness of »0.1 microns on the samples. The proportion of oxygen dissolved in the metal base at temperatures below 450-500°C can apparently be neglected.
In [5], the interaction of titanium with oxygen is described as follows. Through the film of titanium dioxide TiO2 that appears at the first stages of the process, oxygen diffusion occurs to the film-metal interface, where a chemical reaction occurs and further growth of the film thickness occurs. The layer of lower titanium oxides, which should be present between the dioxide layer and the metal, turns out to be very thin and usually does not affect the nature of the oxidation. The rate of diffusion of titanium ions through the film is very small compared to the rate of diffusion of titanium. However, with increasing temperature, the diffusion of titanium increases slightly.
For a short process duration, when the film thickness is still small, the amount of oxygen entering through the film turns out to be sufficient to oxidize all titanium to titanium dioxide. At the same time, as the film thickness increases, the amount of oxygen entering the zone decreases, and the supply of titanium remains constant, since the reaction occurs at the film-metal interface. As a result, when a certain thickness of the scale layer is reached, the ratio of the amounts of titanium and oxygen in the reaction zone becomes such that a TiO layer is formed between TiO2 and the metal. Its appearance weakens the adhesion of scale to the metal, which, under the action of compressive stresses, deforms and peels off, exposing the surface of the metal and providing a sudden increase in the oxidation rate. However, the increased supply of oxygen during scale peeling leads to the oxidation of TiO to TiO2 and the process described above is repeated.
Gas saturation of titanium alloys during oxidation
The interaction of titanium with oxygen is accompanied by two parallel processes: the formation of oxides and the dissolution of oxygen in the metal base.
At temperatures below 8820C and normal pressure, titanium has a hexagonal close-packed lattice - a-Ti. The a-Ti lattice contains four octahedral pores with a radius of 0.414 rat. (0.60 A) and eight tetrahedral pores with a radius of 0.225 r at. (0.36 A). It has been experimentally established that oxygen, the atomic radius of which is 0.60 A, dissolves in octapores. Above 8820C, the structure of titanium is characterized by a body-centered lattice - b-Ti. The b-Ti lattice contains six octapores with a radius of 0.115 rat. (0.22A) and twelve tetrapores with a radius of 0.29 rat. (0.41), that is, the tetrahedral voids in the bcc structure are more spacious. From the standpoint of the geometry of the a- and b-Ti lattices, the dissolution of oxygen is more favorable in the high-temperature modification.
In the resulting diffusion layer, alpha and transition layers are distinguished. The alpha layer differs in structure from the base metal in the increased content of the a-phase, which is easily assessed by metallographic analysis; often this layer is represented by one a-phase. The transition layer does not differ noticeably in microstructure from the base metal, but its presence and penetration depth can be assessed by its higher microhardness compared to the base metal.
Figure 2-Dependence of oxygen diffusion coefficients on temperature:
in a-titanium; 2- in b-titanium.
Gas saturation of the surface of titanium alloy VT6.
In work [6], a study was carried out of the effect of gas saturation on the structure and properties of titanium alloy VT6 in air and in vacuum at temperatures from 750 to 12000C and exposures of 5,30,60,180 and 360 minutes.
The change in microhardness from the surface into the sample depending on the temperature and exposure time is shown in Fig. 3. The microhardness decreases from the surface into the sample under all gas saturation modes.
Figure 3 — Dependence of the microhardness of titanium alloy VT6 on the distance to the surface after heating in air for 1(a), 3(b) and 6(c) hours at 750(1), 950(2), 1050(3), 1200 °C(4).
Heating the VT6 alloy at relatively low temperatures of 750-8000C for 1 hour leads to an increase in surface microhardness from H300 to H400. Increasing the temperature and holding time significantly intensifies the gas saturation process due to an increase in the diffusion rate, as a result of which the surface microhardness greatly increases (Fig. 3). Thus, increasing the holding time from 1 to 6 hours leads at different temperatures to an increase in surface microhardness by H100-200.
With increasing temperature and increasing holding time, the depth of the gas-saturated layer increases (Fig. 4). A gas-saturated layer is formed practically after holding for 1 hour, and a further increase in the duration of gas saturation has little effect on the depth of the surface gas-saturated layer.
Figure 4 - Change in the depth of the gas-saturated layer at different temperatures depending on the holding time.
In [7], the features of gas saturation of titanium alloy VT6 are considered, which are as follows. After exposure to high temperatures and subsequent cooling in air, the VT6 alloy develops cracks that come to the surface. The reasons for their occurrence are internal stresses and reduced plasticity of a particularly fragile gas-saturated layer. The phase composition and properties of the surface layer differ sharply from the composition and properties of the base metal. In particular, the polymorphic transformation temperature of this layer is much higher, the volumetric transformation effect is smaller, and the linear expansion coefficient is greater than that of the base metal. As a result, during cooling, the internal parts of the workpiece undergo less thermal shrinkage and the surface layer is forcibly stretched. The resulting tensile stresses, combined with the reduced plasticity of the gas-saturated layer, lead to the formation of cracks. When measuring microhardness layer by layer, after the gas-saturated zone of increased hardness there is a small section bordering the base metal and having a lower hardness in comparison with it. This is explained by the processes of mutual diffusion of gases from the surface deep into the metal and atoms of the base metal and alloying elements to the gas-metal interface. As a result, the interface between the metal and the gas-saturated layer is depleted of alloying elements and gives a reduced hardness when tested.
Corrosion cracking phenomenon
In a metal subject to corrosion cracking, in the absence of external stresses, very little corrosion damage usually occurs, and in the absence of a corrosive environment, under the influence of stresses there is almost no change in the strength or ductility of the metal. Thus, during the process of stress-corrosion cracking, i.e., under the simultaneous influence of static stresses and a corrosive environment, there is a significantly greater deterioration in the mechanical properties of the metal than would occur as a result of the separate but additive action of these factors. Stress corrosion cracking is a characteristic case where a chemical reaction and mechanical forces interact to result in structural failure. Such destruction is brittle in nature and occurs in ordinary ductile metals, as well as in copper, nickel alloys, stainless steels, etc. in the presence of a certain corrosive environment. When studying the process of brittle fracture as a result of corrosion cracking, of particular importance is the study of the separate effects of stress and the corrosive environment on the metal, as well as their simultaneous effect. However, in the process of corrosion cracking, the following stages are of primary importance: 1) initiation and occurrence of cracks and 2) subsequent development of corrosion cracks. Both stages, as will be shown below, are individual steps in the corrosion cracking process.
The environments in which corrosion cracking of metals occurs are those in which the corrosion processes are highly localized, usually in the absence of noticeable general surface corrosion. The intensity of localized corrosion can be very significant, as a result of which the process of development of very narrow depressions progresses, probably reaching its greatest magnitude at the bottom of depressions having radii of the order of one interatomic distance.
When a material is exposed to a corrosive environment, which affects the susceptibility of the alloy to corrosion cracking and the nature of destruction, the main factors are the following:
1) the relative potential difference of the microstructural phases present in the alloy, which causes the likelihood of local destruction
2) polarization processes in the anodic and cathodic areas
3) the formation of corrosion products that affect the corrosion process.
In order for the corrosion cracking process to occur, the presence of surface or internal tensile stresses is necessary. Failures usually encountered in practice are caused by the presence of residual stresses arising during the production and processing of metal, but for the purposes of the study, no distinction should be made between residual stresses and stresses resulting from applied external loads. Corrosion cracking has never been observed as a result of surface compressive stresses; conversely, compressive surface fracture stresses can be used to protect against corrosion cracking.
As the magnitude of the applied stress increases, the time until complete destruction of the metal decreases. Corrosion cracking usually requires high stresses approaching the yield point, however, failure can often occur at stresses significantly lower than the yield point. For many alloy systems, there is some kind of "threshold" or "limit" of stresses, i.e., stresses below which corrosion cracking will not occur within a certain period of time. This dependence, observed, for example, during delayed cracking of steels, indicates that stresses play the main role in the fracture process.
The most effective method of increasing the resistance of metals against corrosion cracking is to use appropriate design measures and processing methods that reduce the magnitude of residual stresses to a minimum. If residual stresses are unavoidable, heat treatment can be successfully applied to relieve these stresses. If conditions permit, shot peening, for example, can be used to induce compressive surface stresses, which subsequently make it possible to load the material without causing stress on the surface. One method that is gaining increasing acceptance and which is associated with the electrochemical factor of the cracking process is the use of cathodic protection.
Protection of structures and machines made of titanium and its alloys from corrosion
Protection of structures made of titanium and its alloys from corrosion destruction consists of a whole range of measures to increase the performance and reliability of these structures and machines in a corrosive environment. Some of these measures are laid down during the design process, some during the manufacturing of machines or structures, and other measures must be taken during operation.
1) Creation of rational structures. The choice of materials and their combinations for a given product, of course, is dictated by technical and economic feasibility, but must ensure its corrosion resistance. The designer must provide rational forms for machine parts that allow for quick cleaning of dirt; The machine should not have places where moisture accumulates, which is a causative agent of corrosion.
2)Environmental treatment. For different types of corrosion processes, environmental treatment takes different forms. This may include removing or reducing the concentration of substances that cause or accelerate corrosion processes, as well as the introduction of corrosion retarders or inhibitors.
For example, high-temperature gas corrosion occurs mainly due to oxygen in the air or other oxidizing media, from which it is impossible to remove oxygen, since this will disrupt the operation of machines (engines) or structures (shells, planes, etc.). Therefore, treatment is reduced only to the removal of catalytic substances or substances, the presence of which leads to the disruption of stable oxide layers that passivate the metal.
The stability of oxide layers is adversely affected by the presence of halogens that form volatile compounds. Absorption of halogens or changing the composition of the oxidizing environment (halogen-free) significantly increases the stability of metal surfaces.
Environmental treatment can also fully include general measures to preserve the environment, requiring the purification of industrial and exhaust gases, since an increase in the content of SO2, CO2, nitrogen oxides and other gases in the air not only has a detrimental effect on the environment, but also accelerates destruction metal structures as a result of atmospheric corrosion, especially in large cities and near industrial enterprises.
In instrument-making practice, when sealing circuits, air is usually replaced with helium or high-purity argon, which generally eliminates corrosion. If possible, a vacuum of 1.33 • 10-2 - 1.33 • 10-3 Pa is created. If it is necessary to communicate the instrument device with the atmosphere and it is impossible to seal it, absorbers are installed that absorb moisture and carbon dioxide from the air and thereby reduce the possibility of the occurrence of corrosive vapors.
3)Creation of protective coatings. The purpose of their application is to prevent direct contact of the surface of metals and alloys with aggressive components of the environment (H2O, O2, H+, NOx, SO2. SO3, etc.). Such coatings not only provide protection against corrosion, but also impart aesthetic qualities to products ( decorativeness). Protective coatings must be more resistant to corrosion than the metals being protected. Such coatings must be continuous and adhere well to the metal base (good adhesion).
The most important grades of titanium dioxide, application, manufacturers
Features of the occurrence of galvanic pairs
One of the problems when using titanium products can be the occurrence of electrochemical corrosion. There are several main cases in which corrosive galvanic couples may appear:
- Contact with electrolyte. This is true if two metals of different types are used. They can be in a state fastened to each other. There is also a good chance that titanium will corrode more severely if there is contact between metals with different levels of activity.
- When metals come into contact with materials that act as semiconductors. In this case, the free metal can accumulate a negative charge, which becomes positive in the compound.
- Accumulation of electrolyte in the air or contact with it in solution. Electrochemical corrosion of titanium in this state can become even faster and more intense.
It is also worth paying attention to the situation in which the metal is used. In some cases, to ensure optimal protection, it will be possible to simply change the operating conditions or eliminate a potentially dangerous neighborhood.
Physical and mechanical properties
Titanium is a fairly refractory metal. Its melting point is 1668±3°C. In this indicator, it is inferior to such metals as tantalum, tungsten, rhenium, niobium, molybdenum, tantalum, zirconium. Titanium is a paramagnetic metal. In a magnetic field it is not magnetized, but is not pushed out of it. Image 2 Titanium has a low density (4.5 g/cm³) and high strength (up to 140 kg/mm²). These properties practically do not change at high temperatures. It is more than 1.5 times heavier than aluminum (2.7 g/cm³), but 1.5 times lighter than iron (7.8 g/cm³). In terms of mechanical properties, titanium is much superior to these metals. In terms of strength, titanium and its alloys are on par with many grades of alloy steel.
Titanium is as resistant to corrosion as platinum. The metal has excellent resistance to cavitation conditions. Air bubbles formed in a liquid medium during active movement of a titanium part practically do not destroy it.
It is a durable metal that can resist fracture and plastic deformation. It is 12 times harder than aluminum and 4 times harder than copper and iron. Another important indicator is the yield strength. As this indicator increases, the resistance of titanium parts to operational loads improves.
In alloys with certain metals (especially nickel and hydrogen), titanium is able to “remember” the shape of the product created at a certain temperature. Such a product can then be deformed and it will retain this position for a long time. If the product is heated to the temperature at which it was made, then the product will take its original shape. This property is called “memory”.
The thermal conductivity of titanium is relatively low and the coefficient of linear expansion is correspondingly low. It follows from this that metal is a poor conductor of electricity and heat. But at low temperatures it is a superconductor of electricity, which allows it to transmit energy over considerable distances. Titanium also has high electrical resistance. Pure titanium metal is subject to various types of cold and hot processing. It can be drawn and wired, forged, rolled into strips, sheets and foil with a thickness of up to 0.01 mm. The following types of rolled products are made from titanium: titanium strip , titanium wire , titanium pipes , titanium bushings , titanium circle , titanium rod .
Basics of protecting titanium from corrosion development
There are several most common means that can greatly reduce the risk of removing the protective film on the surface of the material.
There are several most common methods:
- Rationalization of the structure of the structure. It is necessary to pay attention to where exactly the part is used, whether there is a potentially dangerous neighborhood that can stimulate the appearance of the process of electrochemical corrosion. It is best that the structure of the product is such that it can be quickly and easily cleaned of accumulated dirt and various potentially hazardous substances.
- Working with the environment. You need to pay attention to whether the environment in which the titanium product is used is dangerous. It is possible to influence the environment using different types of additives. This way, solutions of acids and alkalis can be made less aggressive and the duration of use can be increased without potential external problems.
- Application of a special protective coating to the material. The main thing that such a coating provides is the prevention of metal contact with aggressive environments and oxidation catalysts. It is necessary to pay attention to ensure that such coating maintains its uniformity and integrity throughout the entire period of operation. If necessary, this coating can be further updated.
Our company provides services for high-quality protection of materials from corrosion.
We are ready to answer all customer questions, as well as quickly prepare everything needed to eliminate potential oxidation risks when using titanium products. Return to articles Share article
Types of oxidation
Today a large number of species are used. They are presented in the following categories:
Anodic oxidation
This type is quite common. It is the formation of an oxide film on a metal to prevent corrosion by polarizing their anodes in an environment that is created by connecting an electric current. This method is used for metals such as aluminum, magnesium, titanium.
Microarc oxidation
This procedure consists in the fact that the oxides of many metals, which were obtained by electrochemical oxidation, are subjected to chemical modification using an electric current. Thanks to periodically occurring electrical impulses, a dense film appears on the surface of metals, which serves as reliable protection against corrosion. This procedure is also called plasma electrolytic oxidation. It is used only in a small number of enterprises.
Cold oxidation
This procedure only applies to different types of steel materials. It is also called blackening.
Alkaline oxidation
Today, it is not uncommon to use an alkaline environment for metal processing. Steel surfaces are ideal for this process. The technology of alkaline oxidation involves the production of an alkaline medium so that when interacting with the metal, an oxide film is formed on its surface as a result of the interaction.
Low temperature oxidation
This type of oxide film formation process is neutral. The process uses a method of heating to low temperatures, which ensures that the metal is coated with a weak oxide film.
Electrochemical oxidation
Different types of metals are subjected to this procedure. Metals are immersed in an electrolyte environment.
Table 2. Compositions of solutions for pickling.
| Pickling of aluminum and its alloys | Temperature | Time of processing |
| Composition 1: | ||
| Nitric acid 10-15% solution (by volume) | 20°C | 5-15 s |
Table 3. Compositions of solutions for painting aluminum black.
| To dye black: | g/l (water) | Temperature and processing time |
| Composition 1: | ||
| Ammonium molybdate = ammonium molybdate = ammonium molybdate = ammonium paramolybdate = (NH4)6Mo7O24 | 10-20 | 90-100°C / 2-10 min |
| Ammonium chloride = ammonium chloride = NH4Cl | 5- 15 | |
Table 4. Compositions of solutions for painting aluminum gray.
| To dye gray: | g/l (water) | Temperature and processing time |
| Composition 1: | ||
| Arsenic(III) oxide = arsenic trioxide = arsenic trioxide = arsenic trioxide As2O3 | 70-75 | Boiling / 1-2 min |
| Soda ash = sodium carbonate = sodium carbonate. Chemical formula, Na2CO3 | 70-75 | |
Table 5. Compositions of solutions for painting aluminum green.
| To dye green: | g/l (water) | Temperature and processing time |
| Composition 1: | ||
| Orthophosphoric acid | 40-50 | 20-40°C / 5-7 min |
| Sour potassium fluoride = potassium bifluoride = potassium hydrophoride = kalium bifluoratum = potassium bifluoride = kaliumbifluorid = KHF2 | 3-5 | |
| Chromic anhydride = chromium(VI) oxide = chromium trioxide = CrO3 (very reactive substance, can cause fires and explosions upon contact with organic substances) | 5-7 | |
Table 6. Compositions of solutions for painting aluminum orange.
| To dye orange: | g/l (water) | Temperature and processing time |
| Composition 1: | ||
| Chromic anhydride = chromium(VI) oxide = chromium trioxide = CrO3 (very reactive substance, can cause fires and explosions upon contact with organic substances) | 3-5 | 20-40°C / 8-10 min |
| Sodium fluorosilicate = sodium fluorosilicate = Na2SiF6 | 3-5 | |