What is
In the list of chemical elements, the position “cupronickel” is absent.
Cupronickel is not a metal. This is an alloy of several components.
The basic components of cupronickel are copper and nickel. They are supplemented by iron and manganese, which are added to diversify the color range and consumer characteristics of the product.
Basic properties
Cupronickel is a silvery solid solution that does not exhibit magnetic properties. This material is stronger and lighter than silver, and heats up more slowly than the precious metal.
physical characteristics:
- density of cupronickel - 8900 kg/m3, silver - 10500 kg/m3;
- specific heat capacity at room temperature - 400 J/kg∙°C, silver - 235 J/kg∙°C;
- melting point depending on the composition - 1150-1230 °C, silver - 961 °C;
- temperature coefficient of linear expansion - 16∙10-6 1/°C, silver - 19.5∙10-6 1/°C;
- resistivity - 420 nOhm∙m (depending on the alloy);
- temporary tensile strength - up to 400 MPa, silver - up to 150 MPa (the strength of cupronickel is comparable to the strength of St3 structural steel);
- hardness on the Brinell scale is 65-70, with heating to 300 °C and slow cooling in the oven it increases to 100-120;
- the relative tensile elongation of alloy MN19 is 35%, MNZhMts30−1−1 is 25%.
Increasing the proportion of manganese and iron increases the ductility and conductivity of the alloy. In this case, the material loses its hardness. Key chemical properties of cupronickel :
- high corrosion resistance;
- increased resistance to the destructive influence of both sea and fresh water;
- resistance to atmospheric influences, water vapor, dry gases;
- Cupronickel does not dissolve in salts, does not react with organic acids, and does not oxidize at temperatures up to +150 °C.
Cupronickel and its derivatives are amenable to any technological operations under pressure. Metals are easily cut, stamped, and minted in both cold and hot states.
Products made from cupronickel are polished and soldered. The strength of seams during soldering is increased with special solders made of silver or an alloy of tin and lead.
Health Hazard
Copper, which is part of the alloy, is toxic when heated, so dishes and cutlery made of cupronickel are additionally coated with a protective layer of metal. The inner surface of the dishes is silvered, gilded, tinned with food grade tin, nickel or chrome plated.
Cutlery must be plated with 999.9 standard silver. Such utensils are safe for human health, but the thin coating requires careful care.
Story
The Chinese were the first to obtain nickel silver from metals back in the 3rd century BC. But the alchemists of the Celestial Empire did not discover the secret of “white copper”.
Europeans unraveled the secret two millennia later:
- The French Maillot and Chaurier received the alloy by 1819.
- According to their authentic spelling – Maillot + Chorier – the alloy was christened.
- However, the term entered history modified: the Melchior variant seemed more harmonious to the Germans, and then to other peoples.
Melchior was the name of one of the trinity of wise men who came with gifts to the newborn baby Christ.
- In Russia (USSR), the term took hold by the 1930s, displacing the “German composition”, “serebrovid” and other variants.
- In Europe the term Cupronickel is in circulation.
An alloy that resembles a noble metal in description is also called Nickel silver, German silver, New silver (nickel, German, new silver).
History of origin
Melchior has gone through a long and difficult historical path. The first creator of this alloy is considered to be the Chinese Li Lian Ying, who was a talented master in the field of casting. His abilities were noticed by other masters, and soon Li Lian Ying was invited to the position of court foundry worker. There he made an important discovery. A foundry worker accidentally invented the first cupronickel, which included nickel, zinc and bronze. But the authorities did not thank the master for the invention and even expelled him from the imperial monastery. And the new alloy, which at that time was called pakfong, began to be used for minting coins, making jewelry and various products. At that time, the alloy was very valuable and was not inferior in value to silver.
Europe learned about cupronickel in the 13th century AD. e. Imported products made from this alloy quickly gained popularity. People tried in different ways to find out the composition of cupronickel. As a result of numerous experiments, alchemists still managed to obtain valuable information about the composition of the alloy. But metal production began only at the end of the 18th century in Germany. The Germans made some changes to the composition of cupronickel, replacing bronze with pure copper. This alloy is called “nickel silver”.
The final stage in the history of cupronickel is associated with the French. During the Napoleonic battles, the secret of nickel silver production was stolen. From that moment on, the French began to produce the alloy according to the extracted recipe, but without zinc. The new metal was called “mayshore”, but it never caught on. At the German initiative, the alloy was renamed “cupronickel” in honor of the biblical sorcerer. This name is still used today.
Rafting was especially popular in Tsarist Russia. Products made from this metal were most valued by the middle strata of society. Cupronickel silver, whose composition differed from precious metals, was not recognized in high society. In some circles, nickel silver items were considered fakes.
Physico-chemical characteristics
Each of the components affects the physical and chemical properties of the alloy:
- External resemblance to silver.
- Wear resistance, strength.
- Plastic.
- Resistant to salt water and aggressive environments.
- Biological neutrality, compatibility with the human body.
- Low thermal and electrical conductivity.
The last property of the alloy seems to be a disadvantage, but for dishes or jewelry it is an advantage:
- The dishes do not heat up as quickly or strongly.
- Earrings or a ring do not heat up in the heat, so they do not injure the skin.
There is no single formula for cupronickel; the composition of the alloy is “mobile” (%):
- Copper – 70 – 95;
- Nickel – 29;
- Iron – maximum 0.79;
- Manganese – maximum 0.95.
The formula would look like CuNi , in an expanded version - CuNiFeMn .
There is no single alloy color or melting point. These characteristics are determined by the copper/nickel ratio. The temperature varies from 1145 to 1225°C. The more copper, the more intense the ocher reddishness.
Originally, cupronickel alloy contained zinc, but today it is a component (along with copper and nickel) of another alloy - nickel silver.
Areas of application
An important distinctive property of cupronickel is its electronegativity in contact with sea water. Therefore, cupronickel alloy is used as the main material in the production of parts for submarines. Nickel silver is also used for the manufacture of thermoelements and precision resistors for technology.
The properties of cupronickel allow this alloy to be used for the production of dishes and cutlery. Many modern coins are made from cupronickel alloy. Typically, the alloy composition for coinage is 75% copper and 25% nickel.
Cupronickel is widely used in the jewelry industry. Jewelry made from this alloy is almost impossible to distinguish from silver items. Very often, cupronickel is processed using the filigree technique and decorated with Ural semi-precious stones.
Cupronickel alloy Monel is used for industrial purposes as a structural material.
Stamps
Cupronickel products made in Russia are marked with letters of the Russian alphabet and numbers:
- MN (copper and nickel).
- MNZHMts (plus iron and manganese).
- The numbers show the percentage of nickel, iron, manganese.
The amount of copper in the alloy marking is not indicated.
For example, the combination MNZHMts 45-4-1 is used to brand material made from 45% nickel, 4% iron and 1% manganese. The remainder (50%) goes to copper.
In industry, the most popular alloy grade is MH19. This is 18-20% nickel plus one and a half percent alloys (usually cobalt).
Where is it used?
The properties of cupronickel ensured its use by industrialists, decorators, and bankers.
Industry, medicine
The structure of the alloy allows all types of mechanical processing - cutting, forging, stamping, soldering. And at any temperature.
This determines the areas of application of cupronickel:
- High precision resistors, heat generators.
- Production of components for trawlers, cruise ships, ocean liners. And private yachts of the premium segment.
- In combination with lithium, nickel-iron alloys are purchased for the production of batteries. They are three times more effective than their analogues. Lithium, as the lightest metal, reduces the massiveness of the filling of nickel-zinc or ferrous batteries.
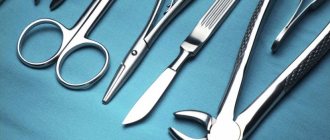
Cupronickel is a material for medical instruments: scalpels, forceps, etc.
Plus the possibility of decorative processing of products (chasing, cladding, polishing).
Decor
Decorators work with alloy in two directions:
- Jewelry. Rings, earrings, necklaces, and cufflinks made of cupronickel are indistinguishable from silver in appearance. However, large companies are not interested in the alloy due to its low cost. It is more often done by hand-made masters. The processing makes them look like museum pieces.
- Dishes. Sets of cupronickel tableware - spoons / forks / knives / sockets / tureens - are a pride and heirloom in many homes. Their advantages: they are several times cheaper than silver ones, but more durable and look presentable.
Especially good are gold-plated, silver-plated, blackened or other coated surfaces.
Applying such a layer is not only a tribute to aesthetics or status. Pure cupronickel imparts tangible metallic notes to food. Copper is toxic, especially when hot.
Banking sector
Wear resistance, coupled with low cost, prompted the National Banks of many countries to issue cupronickel coins.
Almost all silver-colored coins in circulation are made of cupronickel.
In the USSR and Russia, coinage is controlled by GOZNAK:
- In the 1930-1950s, nickels, 10-, 15-, 20-kopeck coins were issued.
Today, the subject of numismatists' hunt is a Soviet patch of the 1937 model.
- Since the 70s, the line has been supplemented with anniversary and regular “rubles”.
The last Soviet coin was the bimetallic 10-rublevik.
In Russia, until the mid-1990s, coins were minted in denominations of 10, 20, 50, 100 rubles. After the redenomination of 1997 - 1 and 2 rubles.
Application area
Stability in sea water has led to the widespread use of cupronickel alloys in the manufacture of components operating in sea water.
The attractive appearance has allowed the copper alloy to occupy a stable niche in jewelry, no longer for counterfeiting silver items, but as an independent alloy. Together with its high wear resistance, cupronickel's attractive appearance has made it the dominant alloy in coin production in different countries.
Nickel silver, which is similar in composition, serves as the basis for the manufacture of precision resistors in electrical and radio engineering.
Both previously and currently, cupronickel is actively used in the manufacture of tableware. Cupronickel spoons are inexpensive and quite widespread. It should be noted that cupronickel silverware, especially forks and spoons, are often coated with a layer of silver. This is not done only for aesthetic reasons or for deception. The fact is that pure cupronickel gives a noticeable and unpleasant metallic taste.
Cupronickel spoons
Cupronickel tableware
The use of copper-nickel compositions in industry has made it possible to improve the performance of product components and at the same time reduce the cost and manufacturability of manufacturing.
Cupronickel, nickel silver, silver: how to distinguish?
The market is flooded with products made from precious metals and budget alloys.
Nickel silver and cupronickel are approximately equal in value and are rarely passed off as each other. The identification symbol is the marking: MN, MNZHMts for cupronickel, MNC for nickel silver.
More often, cupronickel is offered under the guise of a noble material.
Marking allows you to visually distinguish the alloy from silver: legally produced products are always branded. For silver ones these are three numbers, usually 925, for cupronickel ones - the letters MN.
There are other ways to identify cupronickel:
- Draw with a lapis pencil. Only a dark mark will remain on the alloy. With a drop of iodine the effect will be the opposite.
When testing silver, iodine is dripped onto an inconspicuous area: the stain is difficult to remove.
- Thermal conductivity. In a container with hot water, the silver sample will heat up almost immediately, not the cupronickel silver sample.
- Water on the surface . Only on cupronickel, even a drop will leave a greenish stain after a couple of hours. Silver will not be harmed.
- Smell. You can smell the sample. Nickel silver can be distinguished by its copper aroma.
To be sure, it is better to buy goods in stores or from other trusted sellers.
How to use and care
Cupronickel, like fine silver, fades and darkens over time. Even with proper storage, zero humidity in the room is unattainable.
Cleaning
The easiest way to add shine is to buy a special cleaner for cupronickel. Its disadvantage is the need to thoroughly rinse the item after cleaning. Especially dishes.
Generations of owners have tested methods of caring for nickel silver using improvised means:
- Apply a mixture of moistened soda to flannel, suede or other soft fabric. Rub the surface until results are obtained.
- Pour ammonia over the products, then rinse with running water and wipe dry. You can work wearing a mask.
- Boil cupronickel in a decoction of garlic, onion peels or crushed eggshells.
Cleaning with tooth powder, toothpaste, crushed chalk, or products containing bleach is excluded. The first three will scratch the product. The latter will make the cupronickel darken.
Usage
After washing cupronickel dishes after a meal, rinse them with a soda solution (45-50 g per liter of water).
Dishes or other items are immediately wiped dry to prevent them from becoming dull.
Regular thorough cleaning of jewelry or devices will not hurt even if they are used constantly. Resuscitating “neglected” cupronickel will take more time, effort and nerves.
It is better to store products (dishes and jewelry) closed in their “original” packaging. Alternatively, wrapped in cling film or foil. This will minimize mechanical or “wet” effects. That is, they will not be scratched or darken.
How to care for cupronickel products
Products made from cupronickel require special care. So, objects made from this alloy should be cleaned in warm water, adding a few drops of cleaning agent to it. To make the surface of the products shine, you can lightly rub them with toothpaste and then rinse thoroughly.
Cupronickel cannot be cleaned with products that contain chlorine, as it will oxidize the metal. Also, do not use regular cleaning powders. Abrasive products can ruin the polish.
Cupronickel silver cutlery should be stored in a closed box, pre-wrapped in plastic wrap. If the products have been in a damp room for a long time and become stained, you can clean them with a vinegar solution. To do this, you need to dilute 1 tbsp. l. vinegar in a glass of water. Wipe the products with a woolen rag soaked in the solution. After each wash, cupronickel must be wiped dry.