Concept
This term in materials science refers to a mechanical property that determines resistance to destruction under the influence of other, denser substances. Otherwise we can say this: this is resistance to deformation from pressure. In this case, both plastic and elastic changes are taken into account.
Many processes and conditions depend on the characteristics:
- Wear resistance is how long an element can be used. This includes wear and tear, since for every part, such as a car part, there comes a time when, for natural reasons, it needs to be replaced. But the harder the element, the longer it will last under certain conditions.
- Possibility of various types of metalworking - some technologies are applied only to soft alloys, while others can be used for durable ones.
- Resistance to pressure and other forces is characteristic of a shaft or bearing that is subject to centrifugal and frictional forces.
- The ability to use a material as a tool for a more pliable surface. Tool steel is so strong that it is used to make cutters for milling machines, drills and other products.
This is not a complete list of what the hardness of a metal affects after we have defined it. Not every substance used has the same characteristics. What is done first of all to increase this parameter? First, we take raw materials, clean them of impurities, and then subject them to chemical and temperature treatment. Namely: we add various alloying components to the composition that increase this quality, for example:
- Chromium. Strength and corrosion resistance increases, ductility and susceptibility to magnetic forces are slightly reduced. If more than 13% chromium, then the alloy is called stainless.
- Tungsten. The content of solid compounds – carbides – increases greatly. An additional property is reduced brittleness after tempering.
- Vanadium. The resistance to deformation also increases.
- Manganese. To see the effect, the substance must be at least 1%. Impact resistance skyrockets.
What determines the hardness of metals in this class:
- From the presence of alloying additives listed above.
- From the natural properties of raw materials.
- From heat treatment. Hardening helps for this purpose - the material is heated above a certain critical point, the crystal lattice changes, and after cooling, the hardened steel becomes very reliable.
- From cementation - by diffusion, the sample is saturated with carbon. Only low carbon or alloy parts are subjected to this method.
- From aging - it can be natural or artificial. In the first case, processes occur over time that do not affect the microstructure, but are important at the general level. In the second, heat treatment is used to chemically and thermally increase the service life - aging.
- From hardening to the surface. This is a plastic change in the structure of a substance, leading to an increase in strength.
- From laser processing. The laser machine deposits a durable layer.
In addition, some stages of metalworking (rolling, forging and hardening) with changes in the shape of the workpiece also lead to improved quality.
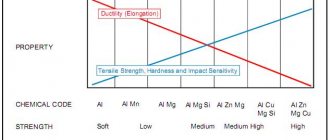
Classifications and properties of various metals
Metals are a group of simple substances that differ from all others in their characteristic properties. Under natural conditions, they cannot be found in their pure form - only as ores and compounds. Their properties are studied by specialists in chemistry, physics, and metallurgy.
When working with metals, it is necessary to take into account a number of their qualities. Mechanical characteristics affect their ability to resist deformation and fracture. The feasibility of using various processing approaches is assessed based on technological properties. Chemical characteristics influence reactions with different substances, and physical characteristics indicate certain reactions to changes in thermal, gravitational and electromagnetic fields.
When classifying, they are based on such properties of solid metals as:
- Hardness, that is, resistance to destruction.
- Strength, or the ability not to change shape, structure, size after exposure to dynamic, static, alternating loads.
- Elasticity, that is, maintaining integrity when changing shape and the ability to return to its original form.
- Plasticity, which involves maintaining shape and integrity under the influence of forces.
- Wear resistance or preservation of product parameters during friction for a long time.
- Viscosity is the ability to maintain integrity under conditions of increasing physical impact.
- Fatigue, that is, the number and period of cyclic impacts that a solid metal can withstand without losing its integrity.
- Heat resistance, or the ability to retain properties even at high temperatures.
The key characteristic of the materials we are interested in is the negative conductivity of electricity. It increases when the temperature decreases, and when it increases, it is partially or completely lost. Secondary characteristics include a characteristic shine and a high melting point. You also need to understand that there are types of metals that are compounds that act as reducing agents during redox reactions.
All properties of solid metals are interconnected, since its other qualities depend on the components of the material. The most famous classification of metals involves the division into ferrous and non-ferrous, but they are also assessed according to a number of other characteristics.
Ferrous metals have a high density, they are characterized by a high melting point and a dark gray color. The main representatives of this group are iron and its alloys. To achieve specific properties from alloys, alloying additives are added to them.
All ferrous hard metals are divided into subgroups:
- Iron: iron, cobalt, manganese, nickel. Most often they play the role of a base or additive to alloys.
- Refractory: tungsten, molybdenum, titanium, chromium. Melting them requires a higher temperature than working with iron. Alloy steels are made from them.
- Rare earths: lanthanum, neodymium, cerium. They have similar chemical properties, but at the same time have different physical parameters. They are used as an additive to alloys.
- Uranium, or actinides: actinium, neptunium, plutonium, thorium, uranium. Their main area of application is nuclear energy.
- Alkaline earths: calcium, lithium, sodium - are not used by themselves.
The black group includes iron alloys with varying proportions of carbon and additional chemical elements such as silicon, sulfur, and phosphorus. Steel and cast iron are actively used in production.
Normally, steel contains up to 2% carbon, it is characterized by ductility and high technological performance. In cast iron, the carbon content is higher and can reach up to 5%. The properties of this alloy can be changed by adding various chemical elements: sulfur and phosphorus increase brittleness, chromium and nickel make cast iron resistant to high temperatures and corrosion.
We recommend articles on metalworking
- Steel grades: classification and interpretation
- Aluminum grades and areas of their application
- Defects in metal products: causes and search methods
Non-ferrous metals are used more often than ferrous metals, since most of them are raw materials for rolled products. In addition, these solid materials have found application in metallurgy, mechanical engineering, radio electronics, high technology and a number of other areas.
According to physical parameters, such metals are classified as follows:
- Heavy: cadmium, nickel, tin, mercury, lead, zinc. In nature they are found in strong compounds.
- Lightweight: aluminum, magnesium, strontium, titanium and others. They stand out from others due to their low melting point.
- Noble: gold, platinum, rhodium, silver. They have high resistance to corrosion.
In general, non-ferrous metals have a low density and melting point, they are also ductile and usually have a white, yellow or red color. Since these materials cannot boast of high strength, they are not used in their pure form, but are made into light alloys for various purposes. They are also used in the production of equipment.
Note that materials belonging to this group have an impressive atomic weight and a higher density than iron.
Copper is actively used in manufacturing as a conductor of electric current. It has a pinkish-red tint, low resistivity, conducts heat well, is characterized by low density, good ductility and resistance to rust.
No less relevant for the production sector are copper alloys, such as bronze (with the addition of aluminum, nickel or tin) and brass (with zinc). Bronze is used for the manufacture of membranes, round and flat springs, worm pairs and various types of fittings. Brass is used to make strips, sheets, wire, pipes, bushings, and bearings.
The group of heavy metals is one of the main reasons for the deterioration of the environment. Toxic substances are discharged into natural water bodies along with wastewater from industrial enterprises. Some substances in this group can accumulate in living organisms.
Mercury is highly toxic; its compounds enter the atmosphere during the combustion of coal at power plants, after which they end up in water bodies along with sediments. This leads to the fact that a high proportion of a hazardous substance gradually accumulates in the body of freshwater and sea fish, as well as other inhabitants of these systems. When consuming such seafood, a person can become poisoned, which often leads to death.
Cadmium is a dispersed and rather rare element, but it also ends up in the ocean along with wastewater from metallurgical plants. Cadmium is found in small quantities in our body, but if this level is seriously exceeded, it destroys bone tissue and leads to anemia.
Lead is found in a dispersed state almost everywhere. But its excess share in the human body leads to health problems.
In addition to hard metals, soft types of metals .
Aluminum has a silvery-white tint, is light in weight, has good electrical conductivity, is resistant to corrosion and is ductile. Due to these properties, this material is widely used in the construction of aircraft, electrical and food industries. Aluminum alloys are also indispensable in mechanical engineering.
Magnesium is quite easily susceptible to corrosion, but is an indispensable material for use in the technical field. Aluminum, manganese and zinc are used in magnesium alloys because they are easy to cut and have high strength. Such compounds are used for the manufacture of camera bodies, motors and other devices.
Titanium is used in the engineering, rocket and chemical industries. Alloys based on it have low density, excellent mechanical properties, are resistant to corrosion and can be easily processed under pressure.
There are a number of hard metals that are rarely found in nature, and their extraction is labor-intensive. We are talking about noble group metals, such as:
- gold;
- silver;
- platinum;
- rhodium.
People learned about gold and its properties back in the Stone Age. This metal occurs naturally in the form of nuggets with a small proportion of impurities, or it can be found in the form of alloys with silver. Gold has high thermal conductivity, low resistance and good malleability; all these properties are especially valued by jewelers.
Silver is inferior to gold in value. In nature, it is most often found in the form of silver ore. Silver is soft, ductile, thermally and electrically conductive.
Platinum was only discovered in the mid-20th century and is considered a rare material. The fact is that it can only be found in deposits in the form of various alloys, which complicates its extraction. The main value of platinum is that it does not react with acids, and when heated it does not change color or oxidize.
Rhodium is also one of the noble hard metals. It has a silver-blue tint, is resistant to chemical attack, is not afraid of temperature changes, but remains fragile and loses its properties under mechanical stress.
In addition, metals are usually divided into hard and soft.
The softest metals include potassium, sodium, rubidium and cesium. This group also includes gold, silver, copper and aluminum. Gold can be found in marine complexes, granite fragments and even in the human body. It can be destroyed when exposed to external factors. Soft silver is used as a material for dishes and jewelry. Sodium is actively used in most industrial sectors. Mercury is the softest metal in the world; it is used in the agricultural, chemical industries, and electrical engineering.
In what units is the hardness of a metal measured?
The peculiarity of this characteristic is that depending on the method by which the measurement was carried out, the classical designation also changes. Since the parameter cannot be classified among the main physical scales, such as distance, speed, mass, force, there is no single standard in the so-called SI system.
If the researcher uses one of the most standard methods proposed by Brinnell, which we will discuss in more detail below, then the result will be written in kgf/mm2, that is, in kilogram-force divided by square millimeter. Using the scale for measuring the hardness of metals, we can talk about classical examples and their indicators in relation to each other:
- iron alloys - on average 30 kgf/mm2;
- copper and nickel compositions – 10 kgf/mm2;
- aluminum, magnesium and their derivatives – 5 kgf/mm2.
So we conclude that iron is 6 times harder than the soft aluminum compound.
The second popular method was invented by Rockwell. According to it, one conditional value (cu) is equal to the displacement of the cone by 2 microns. If it is marked according to this option, then the indexing is first indicated, then one of three letters - A, B, C and a digital value. If you see the hardness of the HB material on the workpiece, then these are Rockwell units of measurement. Parts marked HR can also be marked with an index, and after 1 of three letters:
- A - indicates that the tests were carried out using a diamond cone with an apex angle of 120 degrees under an applied load of 50 - 60 kg.
- B - speaks of a ball of one-sixteenth of an inch, which is directed to the surface under a weight of 90 - 100 kg.
- C - a similar cone is used as with marking A, but the impact is increased at 140 - 150 kg.
Next comes a number that already indicates what kind of dent has formed.
And another option for how steel hardness is measured is numbers plus the letters HV. Vickers proposes this measurement. While using Shor’s method you can see the following records – 90 HSD.
Table "Metals"
The following table shows the groups of main metals:
| Group of metals | Metal |
| Alkaline | lithium, sodium, potassium, etc. |
| Alkaline earth | calcium, strontium, barium, etc. |
| Transitional | uranium, titanium, iron, platinum, etc. |
| post-transition | aluminum, lead, tin, etc. |
| Refractory | molybdenum, tungsten |
| Colored | copper, titanium, magnesium, etc. |
| Noble | gold, silver, etc. |
Metals are ductile and malleable, especially if there is only one electron at the outer electronic level of the atoms: layers of atoms move relative to each other without destroying the crystal lattice (alkali metals, copper, silver, gold). The atoms of the non-plastic brittle metals chromium and manganese have a large number of valence electrons.
The density, hardness, and melting point of metals vary over a wide range and depend on the atomic mass, atomic structure and crystal lattice geometry. The lightest metal is lithium (density 0.53 g/cm3), the heaviest is osmium (density 22.5 g/cm3). Metals with a density of more than 5 g/cm3 are classified as heavy metals, and less than 5 g/cm3 are classified as light metals.
The lowest melting point is mercury (-39 degrees Celsius), the most refractory metal is tungsten (melting point 3410 degrees Celsius.) The atomization energy of tungsten is 836 kJ/mol, and its boiling point is 5930 degrees.
Metals react with both simple and complex substances. As typical reducing agents, metals react with almost all non-metal oxidizing agents (oxygen, sulfur, nitrogen, etc.):
4Al+3O2=Al2O3
Metals also react with complex substances such as oxides and hydroxides, dilute solutions of acids, and alkalis dissolved in water.
Within the same period, metallic properties weaken and non-metallic properties increase; within the same group (in the main subgroup), metallic properties increase, and non-metallic properties weaken
Rice. 3. Metals of the main subgroups.
How Hard Are Base Metals?
Most materials already have certain characteristics, they have long been measured and recorded in tables, while the reports indicate both the initial values of raw iron and after various types of heat and cold metal working. But when adding non-standard and new additives or procedures, it is necessary to re-measure this indicator. But if you are faced with standard alloys, then you should look at the prepared lists.
Tsvetmet
They are softer than black because they do not contain hard inclusions, and they are not subjected to hardening or other heat treatment methods.
Titan is an exception. Here is the technology used by Brinnell:
| Material | Peculiarities | In NV |
| Copper | It has high ductility and low strength. if special impurities are added, new brands are obtained, then the indicator may increase. | 35 |
| Brass | This is a double or multi-component composition that includes copper. but it is more reliable, zinc or tin are additionally included. | 42 – 60 |
| Aluminum | May be soft or hard, with increased or decreased ductility. | 15 – 20 |
| Duralumin | Modern, lightweight, actively used in aircraft construction. There are additives - copper, magnesium, manganese. | 70 |
| Titanium | Very strong non-ferrous metal. | 160 |
Black metals
These are iron and steel, ferroalloys and cast iron. Sometimes vanadium and manganese are included in this category. General characteristics:
- The method of production is the processing of iron ore.
- Increased strength.
- Immunity to mechanical influences.
- High wear resistance.
- Good weldability.
- Low cost.
Therefore, iron is actively used. It is inappropriate to provide a complete list of all brands, so only the main ones:
- Cast iron - 220 HB.
- Tool steel alloys - up to 700 HB, cutting tools are made from it.
- Stainless steel – up to 250 HB.
Mechanical properties of hard metals
The main mechanical properties of solids and their alloys are strength, hardness, elasticity, plasticity, and viscosity.
Based on these properties, the suitability of the material for use in various conditions is judged.
Strength is the resistance to destruction under load.
Hardness is the ability of a metal to resist the penetration of another body with superior hardness into its surface.
Elasticity – the ability to restore shape and size after cessation of exposure. High elasticity is necessary for springs and springs, so appropriate alloys are used for their production.
Plasticity is the property of changing shape and size under the influence of an external load and maintaining the resulting characteristics after the impact ceases. This quality is the opposite of elasticity. The higher the level of ductility, the easier it is to forge, stamp and roll the material.
Toughness is the ability to resist rapidly increasing shock loads. This property is the opposite of brittleness. Ductile metals are used to manufacture parts that are subjected to shock loads during operation. These can be elements of carriages, cars, etc.
To determine the mechanical properties, tensile, bending and other forces are applied to a solid metal.
Mechanical properties are described:
- tensile strength (kg/mm2);
- relative elongation (%);
- impact strength (kgm/cm2);
- hardness;
- bend angle.
To determine these key properties, using special machines, tests are carried out on:
- stretching;
- bend;
- hardness;
- hit.
Tensile test. This is how the tensile strength and relative elongation of the material are determined. Tensile strength is the force that must be applied per unit cross-sectional area of a sample to break it.
How to determine the hardness of a metal using the Brinell method: features
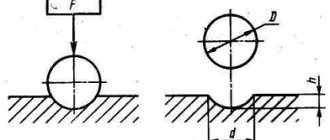
An ideal ball with a diameter of 1 to 10 millimeters is used as an indenter, that is, the element itself that is pressed into the workpiece. It is made from alloy compounds or from an alloy of carbide and tungsten. The production of such balls is regulated by GOST 3722 81.
The time during which static, that is, motionless indentation occurs, is from 10 to 180 seconds. This parameter depends on the material. The shortest time intervals are for cast iron and steel, and longer ones are for non-ferrous metals.
The maximum load that can be measured in this way is 450 or 650 NB, depending on what the ball is made of.
An effect is selected on the sample for the correct deformation; let’s look at the formulas in the table to see how they can be calculated, taking into account that D is the diameter of the ball:
| Object being checked | Mathematical calculated change |
| Lead or tin | 1d^2 |
| Steel connections, titanium, nickel | 30d^2 |
| Light alloys | from 2.5d^2 to 15d^2 |
| Cast iron | 10d^2 or 30d^2 |
| Copper and compositions containing it | 5d^2, 10d^2, 30d^2 |
Algorithm for applying the Brinell method
- The device itself and the body for implementation - the ball - are checked.
- The maximum force is determined.
- The hardness tester starts.
- The depth of indentation is measured.
- Mathematical calculations are performed.
The applied formula is HB=P/F, where:
- P – load;
- F – print area.
It should be noted that this is the most common method.
What is the hardness of steel? (Part 2)
The demand for these methods of measuring metal hardness is explained by their following features:
- all the methods described make it possible to measure each finished sample separately, which undoubtedly improves the quality of serial products;
- there is no destruction of the finished product (for example, a knife) and in the future it can be used for its intended purpose;
- high measurement speed, which means high productivity of the method.
Important: Test results using different methods are not comparable.
Let's consider each method separately, paying special attention to the Rockwell method.
Brinell method
This method was proposed by the Swede Johan August Brinell in the early 20th century. At that time, this was the most accurate way to determine the hardness of metals. Steel balls of various diameters (from 1.2 to 10 millimeters) are used as an indenter. The diameter of the ball is selected depending on the expected hardness of the metal.
Brinell divided metals into several groups, combining them by hardness. The group with minimal hardness included tin, lead and their alloys. The group with the highest hardness included titanium, nickel and steel alloys. For metals with minimal hardness, a ball of the smallest diameter is used; for metals of high hardness, a ball of the largest diameter is used.
Measurements take place according to the following algorithm: the sample being tested is placed on a special table, and an indenter is pressed into the sample from above with a gradually increasing load. This happens over a short period of time from 2 to 8 seconds. After reaching the maximum level of dynamic load, the load is maintained in a static state for approximately 10 seconds. After completing the procedure, the diameter of the indentation is measured on the sample being tested.
Hardness is calculated using a formula that takes into account the applied load, the diameter of the indenter and the diameter of the indentation. Hardness is indicated in the format kgf/mm2, display format HBW.
Vickers method
When measuring hardness using the Vickers method, a pyramid-shaped tip is used as an indenter, the edges of which converge at an angle of 136 degrees. To ensure the accuracy of the test, it is important to observe several points:
- the load must be placed strictly in the center of the diamond tip;
- the load application vector must be strictly perpendicular to the surface of the test sample.
Measurements take place according to the following algorithm: the sample being tested is placed on a special table, and the indenter is pressed into the sample from above immediately with the required load level (the maximum possible value is up to 100 kgf). Next, the indenter is held under load for 10-15 seconds. After removing the indenter, the indentation depth and indentation diagonal are measured.
Next, a calculation takes place according to the form, which takes into account the ratio of the applied load to the diagonal of the indentation and the time during which the test took place.
Hardness is indicated in kgf/mm2 format, display format HV. The Vickers method, due to the use of a diamond tip, allows for more accurate measurements than the Brinell method.
Shore method
This method is a continuation of the well-known “tapping” method, when by tapping a part or workpiece, the master tries to determine its hardness. The method was proposed by the American engineer Albert Shore at the beginning of the 20th century. The essence of the method is that the hardness of the metal is determined by the height of the indenter's rebound.
The device for measuring hardness consists of a hollow tube, on which a cut is made along its entire length with marked divisions. The tube is installed on the surface of the sample being measured and a striker with a diamond tip is dropped into it. The hardness of the metal is determined visually by the rebound height of the striker. Essentially, this device is a “sclerometer”.
This type of measurement does not provide high accuracy, but is excellent for express assessment of the hardness of alloys in metallurgical production, when you need to quickly determine the hardness of a large part or a part that has a complex surface.
Shore hardness display format is HSD (or HSC, depending on the scale used).
Rockwell method
Recently, this method has become widespread due to its simplicity and versatility. The Rockwell method does not require additional calculations and the measurement value is immediately displayed on the instrument scale.
This method was invented by two namesakes who shared the same surname Rockwell. Their names were Hugh and Stanley. Both of them worked in a metallurgical holding in Connecticut, where at that time the issue of operational measurement of the hardness of bearing elements arose. The existing Brinell method did not allow for high-precision measurements, and also did not allow testing on each finished specimen.
The Rockwells came up with a way to measure hardness based on measuring the difference in the depth of penetration of an indenter into a sample under different loads.
Hardness measurement using the Rockwell method occurs according to the following algorithm: the appropriate scale and indenter are selected, the sample is placed on a specially prepared table, a preliminary load of 10 kgf is applied to it, and the load is removed. Next, the main maximum load is applied and the load is removed. The result of the last measurement is the Rockwell hardness value of the metal.
How to measure metal hardness using the Rockwell method: features
If the previous technology is called classical, then this one can be called modern, since it is more automated. The accuracy is much higher and the scope of application is also much higher, since you can work even with very durable materials.
Characteristics of the method:
- The initial pressure is 10 kgf.
- The voltage is maintained from 10 seconds to 1 minute.
- The result is not calculated mathematically, it is displayed on a digital display.
- Different tips are used, depending on this, markings are placed, which begin with the letters A, B, C. We have already indicated in more detail the decoding of the indices, just recall that a steel ball or a diamond cone can act as an indenter.
There are also lesser known and used E, H, K scales with a smaller diameter ball. The procedure is subject to restrictions:
- It is possible to make tests on one workpiece only at a distance of 3-4 units, equal to the size of the testing object, from each other.
- The thickness cannot be less than the depth of penetration of the tip into the steel multiplied by 10.
Rockwell Study Design
The algorithm is similar and even more simplified:
- It is necessary to evaluate the part and check the functionality of the machine.
- Calculate the maximum load.
- Mount the sample and apply primary stress.
- Maintain a certain period of time.
- Record the result indicated on the scoreboard.
Let's see what a hardness tester looks like and how to use it:
The importance of various properties of hard metals when used in production
Mechanical properties. The main requirement that any product must meet is sufficient strength.
Compared to other materials, metals have higher strength, so they are used for the manufacture of loaded parts of machines, mechanisms and structures.
However, strength is not the only important property. Also, many products must have special qualities to ensure their normal operation. Thus, cutting tools require increased hardness, so they are made from tool steels and alloys.
For springs and springs, steels and alloys with high elasticity are used.
Ductile metals are necessary for parts that must experience shock loads during operation.
A property such as plasticity allows us to process the material of interest to us by pressure, that is, forge, roll.
Physical properties. For aircraft, automobile and carriage construction, the low weight of parts is one of the most important qualities, which is why light alloys of aluminum and magnesium are used in this area. Interestingly, the specific strength, that is, the ratio of tensile strength to specific gravity, of some aluminum alloys exceeds that of mild steel.
Fusibility is considered an important property that allows castings to be produced by pouring molten metal into molds. Low-melting metals (such as lead) are commonly used as quenching media for steel. There are complex alloys whose melting point is so low that they can melt in hot water. Typographic matrices are made from them. In addition, they are used in devices that provide fire protection.
Copper and aluminum have high electrical conductivity, therefore they are widely used in electrical engineering and in the construction of power lines. And alloys with high electrical resistance are indispensable as materials for elements of incandescent lamps and electric heating devices.
Thanks to the magnetic properties of solid metals, it is possible to manufacture dynamos, motors, transformers, various communication devices, such as telephones and telegraphs, and a number of other devices.
Thermal conductivity is used in the soldering and welding process of metals.
The coefficient of linear expansion of a number of alloys is close to zero, which makes it possible to use such materials in the production of precision instruments and radio tubes. During the construction of long structures such as bridges, the expansion of metals must be taken into account. In addition, we must not forget that heating two metal parts fastened to each other with different expansion coefficients leads to their bending or destruction.
Chemical properties. Corrosion resistance is a key property of products used in highly oxidizing environments. Such products include grates, parts of chemical machines and instruments. If increased corrosion resistance is required, stainless, acid-resistant and heat-resistant steels are used. In addition, special protective coatings can be applied to them.
Technological properties are of no small importance when carrying out various types of production operations.
Characteristics of the Vickers technique
Another very simple method, which is distinguished by speed and accuracy, but the high cost of equipment. Let's list the features:
- A diamond pyramid with a more obtuse angle is used - 136 degrees at the apex.
- Deformation of more than 100 kgf is not allowed.
- The time taken is very short - from 10 to 15 seconds.
- You can measure the parameters of any material, including especially durable ones, as well as steels that have undergone heat treatment.
Sequence of research
Simplified algorithm:
- Check the surface layer of the part, as well as all equipment.
- Calculate the allowable force.
- Install the sample and secure it.
- Start the device and after 10-15 seconds analyze the result.
Ways to switch between scales
The fact that laboratories use different methods, as well as the fact that there is no single standard, means that one indicator has to be converted to another number system. It should be noted that in all countries one technology is predominantly chosen. But due to active trade turnover, manufacturers encounter unusual markings. So, let's give a table with similar results for different data:
| Indentation diameter – in mm | According to Brinell | According to Rockwell, category A | IN | WITH | According to Vickers |
| 3,9 | 241 | 62,8 | 99,8 | 24 | 242 |
| 4,08 | 217 | 60,7 | 96,6 | 20,2 | 217 |
| 4,2 | 206 | 59,6 | 94,6 | 17,9 | 206 |
| 5 | 144 | 49,9 | 77,7 | – | 144 |
It may be noted that the lists are not particularly accurate, since depending on the measurements, a variety of alloys could be used. The reports will be correct only if the same material was tested in all five methods.
Hardness measurement methods
All methods for determining the hardness of metals use mechanical action on the test sample - indentation of an indenter. But this does not destroy the sample.
The Brinell hardness method was the first to be standardized in materials science. The principle of testing samples is described above. It is subject to GOST 9012. But you can calculate the value using the formula if you accurately measure the imprint on the sample:
HB=2P/(πD*√(D 2 -d 2 ),
- where P is the applied load, kgf;
- D – ball circumference, mm;
- d – imprint circumference, mm. The ball is selected relative to the thickness of the sample. The load is pre-calculated from accepted standards for the relevant materials: iron alloys - 30D 2; copper and its alloys - 10D 2; babbitts, lead bronzes - 2.5D 2.
Symbol of the test principle
Schematically, the Rockwell research method is depicted as follows according to GOST 9013.
Rockwell hardness test method
The final applied load is equal to the sum of the initial load and that required for the test. The device indicator shows the difference in penetration depth between the initial load and the test load h –h0.
The Vickers method is regulated by GOST 2999. It is schematically depicted as follows.
Mathematical formula for calculation: HV=0.189*P/d 2 MPa HV=1.854*P/d 2 kgf/mm 2 The applied load varies from 9.8 N (1 kgf) to 980 N (100 kgf). The values are determined from the tables relative to the measured print d.
The method is considered empirical and has a wide range of readings. But the device has a simple design and can be used when measuring large-sized and curved parts.
The Mohs hardness of metals and alloys can be measured by scratching. Mohs at one time proposed making scratches on the surface of an object with a harder mineral. He classified known minerals by hardness into 10 positions. The first is occupied by talc, and the last by diamond.
After measurement using one method, transfer to another system is very conditional. Clear values exist only in the Brinell and Rockwell hardness ratios, since machine-building enterprises widely use them. The dependence can be observed when the diameter of the ball changes.
| d, mm | HB | HRA | H.R.C. | HRB |
| 2,3 | 712 | 85,1 | 66,4 | — |
| 2,5 | 601 | 81,1 | 59,3 | — |
| 3,0 | 415 | 72,6 | 43,8 | — |
| 3,5 | 302 | 66,7 | 32,5 | — |
| 4,0 | 229 | 61,8 | 22 | 98,2 |
| 5,0 | 143 | — | — | 77,4 |
| 5,2 | 131 | — | — | 72,4 |
As can be seen from the table, increasing the diameter of the ball significantly reduces the readings of the device. Therefore, machine-building enterprises prefer to use measuring instruments with the same type of indenter size.
If you find an error, please select a piece of text and press Ctrl+Enter.