04/20/2019 Author: VT-METALL
Issues discussed in the material:
- Why does corrosion of metal products occur?
- What is corrosion of metal products?
- How to prevent corrosion of metal products with non-metallic coatings
- What are other ways to prevent metal products from corrosion?
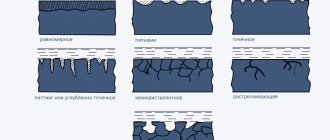
The interaction of metals with the environment leads to their destruction under the influence of electrochemical or chemical processes. In the first case, dissolution occurs due to the aquatic environment or moisture from the air, and in the second, the process of forming compounds with aggressive substances takes place. Corrosion of metal products can form in local areas (local), cover the entire surface of the part (uniform), or spread along the boundaries of crystals (intercrystalline).
Types and causes of corrosion of metal products
The interaction of metals with the environment leads to their destruction under the influence of electrochemical or chemical processes. In the first case, dissolution occurs due to the aquatic environment or moisture from the air, and in the second, the process of forming compounds with aggressive substances takes place. Corrosion of metal products can form in local areas (local), cover the entire surface of the part (uniform), or spread along the boundaries of crystals (intercrystalline).
Oxygen and moisture contribute to the formation of loose brown powder (Fe2O3•H2O) on the surface of the metal, which is called rust.
- Chemical corrosion
In this case, the destruction of metal products occurs in environments that are not conductors of electricity (dry gases, oil products, alcohols and other organic liquids). Chemical corrosion accelerates with increasing temperature and leads to the formation of an oxide film.
All metals, without exception, are subject to this process. The most active of them, such as aluminum, as a result of corrosion, form an oxide film that protects the metal product from deep oxidation. A coating called patina forms on the surface of copper and some low-active metals. It should be noted that the oxide film can serve as protection against further oxidation only if its crystal chemical structure is consistent with the structure of the metal. In other cases, such a film will not protect the metal product from further oxidation.
The corrosion process in metal alloys proceeds somewhat differently. Certain elements of such compounds are reduced instead of oxidizing. For example, at high temperatures and pressures in the structure of steels, carbides are reduced by hydrogen, so the materials lose their characteristics.
- Electrochemical corrosion
For this process to occur, it is not necessary to immerse the metal in an electrolyte. Electrochemical corrosion can occur under the influence of a thin electrolytic film formed on the surface of the metal. In addition, electrolyte solutions can be present in the environment that surrounds the metal product (in the ground, in concrete, etc.). Most often, electrochemical corrosion is provoked by the use of potassium and sodium salts to combat icing of roads and sidewalks in winter. Cars and various engineering communications suffer greatly from such corrosion. Losses caused by the use of salts in the United States after each winter amount to about $2.5 billion.
Extracts from a classic book
I.L. Rosenfeld, V.P. Persianseva “Atmospheric corrosion inhibitors”, M., “CHEMISTRY”, 1985.
Factors influencing the rate of atmospheric corrosion
The most important factors determining the rate of atmospheric corrosion are: atmospheric humidity; atmospheric composition; the total residence time of the moisture film formed on the metal surface; its chemical composition; air temperature.
Humidity of the atmosphere. Absolute air humidity (the amount of water vapor per unit volume of air), other conditions being constant, determines the thickness of the adsorption film formed on the metal surface. So, for example, the thickness of the layer of moisture formed on the surface of iron at a relative air humidity of 55% is 15 molecular layers, and at a relative humidity of 100% - 90 - 100 molecular layers...
The amount of water that condenses on the surface of the metal when the product is cooled depends on the relative humidity; however, even in this case, with its subsequent evaporation, corrosion at lower relative humidity, for example at 76%, may be higher than at 100% humidity. The effect of self-stirring is felt... Low corrosion rates are observed only at relative humidity levels up to 60%. Exceeding this humidity with any amount of moisture condensed on the metal surface leads to a sharp increase in the corrosion rate. However, when there is a large amount of condensed moisture, corrosion at 80% relative humidity is higher than at 100%.
The amount of moisture condensed on the metal surface depends on the temperature difference: the higher it is, the more water condenses at a given humidity...
The amount of condensed moisture can also vary due to the presence of foreign substances on the metal surface. Their influence affects capillary condensation. In addition, they can become centers of crystallization. Substances that are highly hygroscopic are especially dangerous. In their presence, the values of relative air humidity change, at which a sharp increase in the rate of corrosion of metals is observed (Hk - critical humidity) ... So, for example, if there are ammonium salts on the surface of iron, the corrosion rate increases sharply, and air humidity drops from 80 to 50% ...
The nature of the iron corrosion products also has a strong influence on the critical moisture content; for iron coated with corrosion products formed in distilled water, the critical humidity is 65%, and for sea water the critical humidity is reduced to 50% [194]…
In addition to reducing the critical humidity, foreign particles deposited on the metal surface can increase the rate of destruction of metals due to changes in the structure of protective films, as well as their adhesion to the metal surface.
The emergence of surface tension between solid particles and corrosion products can lead to the latter sticking to the solid particle rather than to the metal surface, thereby weakening the protective properties of the resulting corrosion products. This is also observed if the nature of the particles is such that they can interact with metal ions passing into solution to form soluble corrosion products instead of insoluble metal hydroxides.
This leads to a very important conclusion: corrosion products that have arisen for any reason, even in the form of small foci, should be removed as quickly as possible so that they do not contribute to the further development of corrosion. You should also avoid getting foreign particles on the surface of the product.
Composition of the atmosphere. The aggressive properties of the atmosphere in relation to metals are determined not only by humidity, but also by the contaminants that enter it. The most unfavorable types of pollution are sulfur dioxide and sodium chloride. The first enters the atmosphere along with products formed during the combustion of sulfur-containing fuel, the second - due to salt carried by the wind from the surface of the oceans and seas.
Sulphur dioxide. From a certain critical concentration, sulfur dioxide greatly increases the rate of corrosion of metals such as iron, aluminum, zinc, copper and others... At a constant concentration of sulfur dioxide in the atmosphere, the rate of corrosion of metals increases with increasing relative humidity of the atmosphere... An increase in the rate of corrosion of metals is also observed in that case , when there is no sulfur dioxide in the atmosphere, but the surface of the metal has previously been exposed to it. In this case, corrosion proceeds as if the atmosphere contains sulfur dioxide... such a negative effect is due to the fact that sulfur dioxide, in the presence of adsorbed moisture on the metal, forms crystalline hydrates, which are not removed from the metal surface even during pumping and contribute to the emergence and development of the corrosion process. The most dangerous thing in an industrial atmosphere is the deposition of coal dust particles on the surface of a structure...
Sodium chloride. Sodium chloride, like sulfur dioxide, very noticeably increases the corrosion of a number of metals in atmospheric conditions... The main reason for the acceleration of corrosion by sodium chloride is the formation in its presence of soluble corrosion products instead of insoluble hydroxides that arise under a clear film of moisture. In addition, chlorine ions prevent the formation of passivating films. The aggressive action of sodium chloride in atmospheric conditions should also be associated with its ability to adsorb moisture from relatively dry atmospheres. Already at a relative air humidity of 70%, atrium chloride adsorbs moisture, which is accompanied by a strong increase in the corrosion rate...
Of other air pollutants, the most aggressive are chlorine, ammonia, hydrogen sulfide and carbon dioxide... Without dwelling in detail on the influence of these atmospheric pollutants on the mechanism and speed of corrosion processes, we note what is common in the action of these compounds, as well as some of their distinctive features.
A distinctive feature of chlorine is its aggressiveness in both humid and relatively dry atmospheres (H = 42%)... At high relative humidity, a sharp increase in the corrosion rate occurs. The latter is due to the fact that chlorine is a strong cathodic depolarizer at high relative humidity. In addition, as a result of the formation of chloride compounds, it shifts the critical humidity to lower values.
The corrosive activity of hydrogen sulfide, like sulfur dioxide, occurs only when a certain value of the relative humidity of the atmosphere is reached... In a dry atmosphere in the presence of hydrogen sulfide, the corrosion of zinc, cadmium, tin, aluminum, antimony, bismuth, chromium, iron, cast iron, alloy steels, cobalt and nickel is negligible ... Common to the types of pollution under consideration is their lower (in most cases) aggressiveness compared to sulfur dioxide and sodium chloride. Ammonia itself is not dangerous for iron and alloys based on it. However, for copper alloys it poses a great danger, causing corrosion cracking.
Duration of residence of a film of moisture on the surface of the metal... in atmospheres not contaminated with noticeable amounts of specific corrosive impurities, the process of atmospheric corrosion arises and develops only if there is a film of moisture of a certain thickness (10-20 molecular layers) on the surface of the metal, acquiring electrolyte properties. The duration of development of the corrosion process and the amount of metal turned into corrosion products depends on the duration of residence of the electrolyte film on the metal surface. The longer the film formed on the metal does not dry out, or the more often it is renewed, the longer the corrosion process proceeds, and therefore, all other things being equal, the greater the corrosion destruction of the metal...
The significant importance of the duration of the total residence time of the moisture film for corrosion destruction is also confirmed by the fact that, for example, in Batumi, where the maximum amount of precipitation falls in the Union (the number of days with dew is 68) and where, it would seem, corrosion should be the greatest, it turns out to be lower than, say, in the coastal conditions of Murmansk, where the amount of precipitation is significantly less than the number of days with dew - 25). This is explained by the fact that in Batumi, where there are many sunny days, the conditions for removing film from the surface of the metal are more favorable than in Murmansk. Therefore, the total time of contact of the metal with the electrolyte in Batumi is much less than in Murmansk, and therefore there is less corrosion there. The same effect can explain why the side of the sample or structure that faces the ground is more corroded, and not the side that is directly exposed to precipitation.
Air temperature. The rate of corrosion usually increases with temperature, since with increasing temperature the kinetics of electrochemical reactions causing the corrosion process increases.
With atmospheric corrosion occurring ... in visible layers of electrolytes, most often with cathodic limitation, temperature changes the rate of the process mainly due to changes in the kinetics of the cathodic reaction of oxygen reduction, the rate of which is determined by the rate of oxygen diffusion to the electrode.
Since the diffusion coefficient of oxygen increases with temperature (the diffusion coefficient changes according to the law D = RT / (6p rh),
where R is Boltzmann’s constant; r is the radius of the diffusing particle; h is the viscosity of the medium), and the thickness of the diffusion layer decreases (due to increased self-stirring caused by convection), then an increase in temperature should lead to an increase in the limiting diffusion current and, consequently, the corrosion rate. It is necessary, however, to keep in mind that when metals corrode in the atmosphere, the duration of contact of the electrolyte with the metal changes with a change in temperature. Therefore, the overall corrosion effect depends on changes in the kinetics of electrode reactions and on changes in the residence time of the electrolyte on the metal surface.
Due to the presence of two factors acting in exactly the opposite direction (the duration of contact of the metal with the electrolyte decreases with increasing temperature, and the rate of reactions causing the corrosion process increases), the dependence of the rate of atmospheric corrosion on temperature is very complex and is not always easy to predict. If the effects resulting from keeping the metal in contact with the electrolyte for a long time exceed the effects resulting from a more intense process at a higher temperature, then corrosion in areas characterized by relatively low temperatures may be greater than in areas characterized by high temperatures. However, high temperatures, combined with prolonged exposure of the metal to the electrolyte, which, for example, is observed in humid tropical climates, contribute to increased corrosion
Electrochemical method of corrosion protection of metal products
Classic methods of preventing metal products from corrosion are based on electrolytically coating the product with pure metal or a special alloy. The table shows the most popular coatings that can be applied using electrochemical processing.
This method of anti-corrosion protection of metal products has been well studied and can be used for most metals and various alloys. The protective coating is applied to the electrically conductive substrate. The technology of this process provides the ability to regulate the thickness and characteristics of the coating by changing the electrolyte composition, current and temperature parameters. With strict adherence to technological requirements, high quality protective coating is ensured. If necessary, the electrochemical method of protecting metal parts allows you to obtain mirror surfaces without additional polishing.
The cost of equipment for such processing is quite affordable.
We recommend articles on metalworking
- Steel grades: classification and interpretation
- Aluminum grades and areas of their application
- Defects in metal products: causes and search methods
This technology for applying protective coatings is not characterized by high productivity (on the order of 10 - 50 μm/hour per m2), which is due to the low level of use of continuous techniques and the introduction of technological lines for processing rolled materials. In the Russian Federation, rolled steel with electrolytic coating is under the brand name "Polistil" Only the Listvyansky Metallurgical Plant produces in series.The product catalog of this enterprise includes thin-sheet galvanized steel of the EOTs, ETs grades, leaded rolled EOS, chrome-plated tin and other types of products with electrochemical coatings.
This technology for anti-corrosion protection of metal is used by many domestic companies that carry out production activities in various fields.
Corrosion is one of the main problems in the operation of metal structures
The problem of corrosion is much more serious than it is often given credit for. Its relevance is especially evident in enterprises that use metal structures, equipment, machinery, tools and vehicles with significant wear and tear. Corrosion processes have different natures of their occurrence, but they all have one thing in common - they appear as a result of contact and interaction of metals with the environment (physical, chemical and chemical). It mainly occurs in liquid and gaseous media. What happens to metals that fall into the so-called “corrosion” zone? The fact is that these materials have insufficient thermodynamic stability to certain substances with which they interact. The manifestation of corrosion is its products, such as rust.
This process has an extremely negative effect on the equipment and leads to the destruction of tasks and various structures. Such a problem causes enormous damage to the economy of the Russian Federation, which is almost impossible to assess. According to experts, losses from corrosion can range from 3 to 5% of the GDP of countries with developed industry. Moreover, rust and other corrosive effects literally “eat” the metal. Its losses, according to experts, can reach up to 20%. Corrosion can imperceptibly and covertly destroy the strength of materials and lead to serious accidents and emergencies, including loss of life. Restoring structures that have become unusable due to corrosion and replacing equipment for the same reason costs their owners enormous amounts of money.
The problem of corrosion at hazardous production facilities can provoke accidents and emergencies that are fraught with serious environmental problems, as well as accidents causing harm to the health and life of personnel.
Protection of the material part of industrial enterprises from rust and other corrosive processes is a pressing issue today in terms of ensuring industrial safety.
To do this, specialized specialists must clearly understand the processes of rust formation, have knowledge about the physical and chemical properties of metals and methods of protection against corrosion, which is present wherever metal products are processed and used. You can and should fight it. It is much easier to prevent corrosion processes than to eliminate them later.
One of the most effective ways to protect metal structures, and for a long time, is to cover its surface with a thin layer of galvanized film, which prevents oxygen from penetrating into the metal. Namely, its presence leads to rust.
According to statistics, there are 4 kg per Russian resident. zinc coated metal. The same figure in the West is 15 kg. Such information is extremely indicative and reflects the problematic nature of this topic in Russia, where a huge amount of metal is practically not protected from rust and its consequences. This leads to the fact that the shelf life of equipment and metal structures is sharply reduced and at a certain point becomes dangerous for their further operation.
Safety experts are sounding the alarm about the use of metal structures and fittings without anti-corrosion coating in the construction of residential buildings. For example, in the West this is strictly prohibited. This practice is vicious and dangerous. After approximately 5 years, the reinforcement unprotected from corrosion located in the concrete inside a residential building becomes covered with rust. Even a professional in this field cannot assess the actual safety of such buildings.
Experts say the problem is systemic. Currently, there is not a single plant in the Russian Federation that produces special equipment for hot zinc coating of metal surfaces. But, since the need for this technological operation exists, Russian enterprises are forced to purchase similar equipment abroad. And here a new problem arises, which is that old, worn-out equipment is purchased at high prices and with unnecessary additional functions. Considering that there are practically no domestic qualified personnel in this area, the technological process is accordingly disrupted. There are precedents when metal structures become covered with rust, being galvanized, as soon as they reach the construction site.
But it cannot be said that this problem is not being addressed at all in Russia. There are already domestic developments and technologies of Russian scientists in the field of anti-corrosion coating, which are able to protect metal structures from rust for a long time. In addition, this equipment is more efficient in performance and at a much lower price. For example, the new unique technology of domestic scientists for applying aluminum to steel surfaces (rolled steel) is in great demand. According to experts, this technology is an innovative breakthrough in the field of anti-corrosion coating. The fact is that aluminum coating is much more effective than galvanized coating, and significantly (5-10 times). This metal is more stable at high temperatures than zinc. The advantage of aluminum coating also lies in the fact that it does not destroy its internal structural bonds in so-called aggressive environments, and at temperatures reaching 900C. The advantage of this metal compared to its competitors is also in its weight and consumption rates for applying the substance to its surface.
But the expert community in this area believes that it is impossible to solve the problem of promoting and introducing domestic technologies and developments into industrial production without the participation of the state. And this process, in their opinion, needs to begin with the formation of a new legislative framework that will allow the development of innovative developments in the field of anti-corrosion coatings and stimulate industry to implement them.
If you find an error, please select a piece of text and press Ctrl+Enter.
Prevention of corrosion of metal products by hot-dip galvanizing
Hot galvanizing can be called the simplest, but at the same time the oldest method of protecting metal from building structures, angles and wires.
- Surface preparation of metal products
Before applying molten zinc to the surface of steel parts, it is necessary to carry out some preparatory work. The degree of adhesion of the coating to the metal depends on how high-quality the preparation of the products is. Preparatory work is carried out in several stages. First, the metal surface is degreased, then washed, etched and fluxed.
Thorough degreasing of the surfaces of metal products is necessary to remove oil stains and various contaminants. Acids and alkaline solutions are often used for this operation. Depending on the nature of the contamination, certain reagents can be used for degreasing. This operation is carried out at temperatures between 60 °C and 80 °C. After this, the metal product must be thoroughly rinsed to remove any remaining reagents, foam, grease, etc.
At the next stage, the part is etched. To remove rust and scale residues, the product is immersed in an HCl solution with a concentration of 120 - 210 g/l. This operation ensures effective cleaning of metal parts, which is a necessary condition for ensuring adhesion of the zinc coating to the metal. At the same time, to prevent the acid from destroying the workpiece, special inhibitors are included in its solution to prevent the absorption of hydrogen (hydrogenation).
After etching is complete, the part must be rinsed again to remove any remaining solution. For more economical water consumption and ease of washing, sequential baths are used.
Upon contact with water, oxides form on the surface of metal products, which are removed by fluxing. This operation allows you to completely clean the metal and obtain a passive film layer on its surface that protects against oxidation and ensures good adhesion to zinc.
For fluxing, a composition including NH4Cl and ZnCl2 is used. For example, fluxes containing 55.4% ammonium chloride, 6% glycerin and 38.4% zinc chloride are often used in production. This procedure is performed with a concentrated solution (400 - 600 g/l) at 60 °C. In this case, it is necessary to constantly monitor the composition of the solution and promptly clean the bath (to do this, add H2O2 to it). When hydrogen peroxide is added, Fe3+ salts settle in the bath, which are collected in special settling tanks and filtered.
- Drying metal products before hot galvanizing
After fluxing, the part must be thoroughly dried. Otherwise, the remaining water on the surface of the metal, when immersed in molten zinc, begins to evaporate, which leads to micro-explosions and violates the integrity of the zinc layer. In addition, drying parts will reduce the consumption of thermal energy to maintain a stable temperature of molten zinc. The duration of the drying process exceeds the duration of galvanizing itself. To dry, metal products are placed in a drying oven heated to 100°C.
- Hot galvanizing
To ensure reliable protection of metal parts from corrosion, it is necessary to ensure the following components: high quality zinc and metal, accurate temperature control, correct preliminary preparation of the product, the required immersion/lifting speed and a certain duration of immersion, compliance with the requirements for cooling conditions.
When immersed in molten zinc, the flux melts, providing the necessary surface wettability. Dipping too slowly results in the flux melting too early and oxides appearing on the surface of the metal product. Due to too rapid immersion, the flux will not have time to melt, and the zinc coating will have defects. Therefore, it is important to strictly comply with the requirements for the speed of immersion of the product in the bath.
Hot-dip galvanizing technology involves keeping metal products in a bath of molten zinc for 3 to 10 minutes. During this time, a layer of slag forms on top of the melt. Before removing the part, it is necessary to clean it of this slag using a special scraper. Otherwise, it will settle on the galvanized product.
The speed at which a metal part is removed from molten zinc affects the thickness of the protective layer (a thicker coating is obtained with slow extraction). This is related to the rate of crystallization. The duration of lifting and tilting of the product is determined individually, based on its shape and size. The drying process of a part after hot-dip galvanizing is carried out in the open air.
To remove and further utilize HCl vapors and other harmful substances, high-power ventilation systems are installed above all baths of the production line.
The latest lines that perform the hot-dip galvanizing process operate fully automatically. Older equipment is controlled by operators using control panels, which prevents workers from coming into direct contact with harmful fumes.
Advantages of protecting metal products from corrosion using hot-dip galvanizing technology:
- a coating characterized by high corrosion resistance is formed on the surface of the part;
- affordable price;
- simplicity of the technological process;
- ease of equipment maintenance;
- high performance;
- the coating formed after hot-dip galvanizing provides protection of metal products even from mechanical damage;
- the resulting coating is characterized by high electrical and thermal conductivity;
- The hot-dip galvanizing coating prevents the metal from becoming brittle due to exposure to atomic hydrogen.
Disadvantages of technology:
- the size of parts that can be processed using the hot-dip galvanizing method is limited by the dimensions of the bath;
- galvanized metal products are poorly susceptible to further processing and welding;
- relative unevenness of the zinc layer;
- It is impossible to obtain very thin coatings using hot-dip galvanizing;
- relatively high consumption of zinc.
This technology allows you to create a protective layer on the surface of metal products from several microns to one millimeter.
Pipeline corrosion - causes and consequences. Part 1. Main pipelines
Pipeline transport is the most common method of delivering liquid and gaseous media in the world. Small internal pipelines are present in every modern home; networks of above-ground and underground distribution pipelines have been built in populated areas; all regions of our country are connected by a system of main pipeline transport. Pipelines transport water, oil and petroleum products, gas, etc. Our country even built a unique ammonia transport pipeline. The majority of domestic pipelines in use are metal, the main reason for their destruction is corrosion, and there are many types of corrosion. In this review, we will briefly look at the main types of pipeline corrosion depending on their purpose, and also talk a little about the consequences of corrosion accidents.
The causes of corrosion are always determined by the properties of the corrosive environment with which the internal and external surfaces of the pipeline come into contact. Corrosion of the internal surface of pipelines occurs mainly when pumping aqueous media, especially if corrosive substances are dissolved in them: salts, acids, alkalis, etc. This situation occurs in all water pipelines, in particular in heating and hot water supply systems, oil collection systems (remember that in the Russian Federation, oil extracted from wells contains up to 99% water!), and wastewater from industrial enterprises. The most dangerous last case. Corrosion of the outer surface depends on the method of laying the pipeline and the design solutions used. For example, when laying using the “pipe-in-pipe” method, corrosion of the outer surface does not occur. When laying a pipeline in air, atmospheric corrosion occurs, which practically does not lead to a violation of the integrity of the pipeline (the formation of through defects). We have already devoted a separate review , so we will not return to this issue in this article. The main danger of corrosion destruction of the outer surface of pipelines occurs during underground installation, and the purpose of the pipeline in this case is not very important. With few exceptions, all types of underground pipelines corrode equally. A separate issue is underwater corrosion of pipelines laid along the bottom without being buried in the ground. True, this very specific type of corrosion concerns only a few objects in the Russian Federation - offshore gas pipelines, for example, Blue Stream and Nord Stream, as well as several field pipelines in the Caspian, Black and Northern seas. Due to the extremely narrow circle of interested parties and the specificity of the processes, marine corrosion of the outer surface of pipelines will also not be addressed in this review.
So here we go. In this review, divided into several parts, we will separately and in detail consider the possible corrosion mechanisms of the following types of pipelines, classified according to their functional purpose:
- main pipelines;
- field pipelines of oil and gas fields;
- pipelines of heating systems, hot and cold water supply;
- industrial waste water pipelines.
Let's start with, perhaps, the most simple objects from a corrosion point of view - main pipelines for transporting oil, gas, ammonia, petroleum products, etc.
Corrosion of main pipelines
Perhaps this is the most well studied and systematized type of pipeline corrosion. At least, main pipelines are the only type of pipelines whose corrosion protection is regulated by a separate national standard GOST R 51164-98* “Main steel pipelines. General requirements for corrosion protection." Of course, GOST R 51164-98* is mainly devoted to methods of anti-corrosion protection, and not to mechanisms of corrosion destruction, however, upon careful study it is possible to identify a certain systematization of the danger of corrosion of main pipelines depending on its mechanism. It should be immediately noted that main pipelines are used for transporting prepared corrosive-inert products, therefore only external corrosion poses a danger to them, and in areas of overhead installation only relatively harmless atmospheric corrosion
. Further, our review will focus only on sections of underground main pipelines.
So, GOST identifies 3 types of sections of main pipelines that are subject to special corrosion hazards: areas of high corrosion hazard, areas of increased corrosion hazard and corrosion-hazardous areas. Among the GOST criteria relating to corrosion mechanisms and allowing some sections of pipelines to be classified as particularly dangerous areas, the following criteria for areas of increased corrosion hazard can be identified:
- stray currents from direct current sources;
- microbiological corrosion;
- stress corrosion cracking.
Additionally, GOST classifies as areas of increased corrosion hazard the areas where main pipelines are laid, where the risk of ordinary soil corrosion can sharply increase:
- pipeline sections in saline soils of any region of the country (salt soils, solonetzes, solods, sors, etc.);
- sections of pipelines in areas of industrial and domestic wastewater, garbage and slag dumps;
- sections of pipelines with the temperature of the transported product above 303 K (30 °C).
Summarizing the above, as well as many years of experience in operation and diagnostics, we can summarize that the following types of corrosion destruction are mainly realized on underground main pipelines:
- soil electrochemical corrosion;
- corrosion by stray currents from direct current sources;
- corrosion by stray currents from alternating current sources (in areas of intersections and, less often, approaches to overhead lines of 110 kV and above);
- stress corrosion cracking (typical mainly of main gas pipelines);
- microbiological corrosion (in areas where the soil around the pipeline is contaminated with microorganisms).
Soil electrochemical corrosion
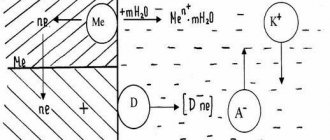
Corrosion of underground pipelines proceeds through an electrochemical mechanism, based on the occurrence of a potential difference between different sections of the pipeline, and, as a consequence, the occurrence of a corrosion current. As a result of the flow of corrosion current, sections of metal in the anodic zones dissolve and pass into the soil, where they subsequently interact with the soil electrolyte, forming rust.
Mechanism of electrochemical corrosion
One of the most important features of pipelines from a corrosion point of view is their large length. Given their great length, underground lines pass through soils of different composition and structure, different humidity and aeration. All this creates the possibility of significant potential differences occurring between individual parts of the underground line. Since pipelines have high conductivity, corrosive galvanic couples are easily formed on them, sometimes extending for tens or even hundreds of meters.
Since this often creates high current densities in the anodic areas, this greatly increases the corrosion rate. It is also important for the development of corrosion that underground lines are laid at such a depth where some moisture is always retained, ensuring the occurrence of corrosion processes. At the depth of pipelines, the temperature rarely drops below 00C and this also promotes corrosion. The development of corrosion on underground pipelines is also facilitated by the presence of mill scale on the surface of the pipes, which is not always removed during cleaning.
It was established that there is a direct relationship between the area exposed to corrosion and the depth of corrosion destruction. This is explained by the fact that on a larger metal surface there is a greater possibility of creating more severe corrosive conditions. In particular, this explains that other steel underground structures, in addition to pipelines, ceteris paribus, are destroyed by electrochemical corrosion more slowly.
The corrosive aggressiveness of the soils themselves is determined by their structure, granulometric composition, electrical resistivity, humidity, air permeability, pH, etc. Typically, the corrosive aggressiveness of the soil in relation to carbon steels is assessed by the electrical resistivity of the soil, the average density of the cathode current when the electrode potential is shifted by 100 mV more negative than the corrosion potential of steel, the gradient of natural free corrosion potentials along the pipeline section.
Corrosion by stray currents from DC sources
Stray currents are currents of anthropogenic origin that flow in the ground and in underground metal structures. Such currents arise due to leakage into the ground of the currents of operated devices and structures operating on direct current, in particular direct current railways, electric welding machines, cathodic protection systems of third-party objects, etc., etc. As is known, electric current always tends to move along the path of least resistance, therefore, if there are long underground metal pipelines in the distribution zone of stray currents in the ground, the electrical conductivity of which is several times greater than the electrical conductivity of the soil, the stray current will flow precisely through them. In the most favorable place (from the point of view of the same principle of least resistance), the stray current will flow from the pipeline back into the ground and return to its source. In this case, the section of the pipeline from which the stray current enters the ground is the anode, and the part of the pipeline where the stray current enters it is the cathode. In the anode sections, stray currents of increased density cause significant corrosion damage to pipelines; the corrosion rate on them is practically unlimited and can reach gigantic values of 10-20 mm/year.
Corrosion by stray currents from AC sources
This type of corrosion occurs in places where overhead lines with a voltage of 110 kV and higher and main pipelines come together and run in parallel. This phenomenon has already been covered in detail on our website in a special review.
and will not be considered further in this article.
Stress corrosion cracking (SCC) or stress corrosion
Stress corrosion cracking in main pipelines (mainly gas pipelines) develops as a result of simultaneous exposure of the metal to a corrosive environment and tensile stresses. Thanks to the research carried out, a hydrogen-corrosion theory of the development of SCC in pipelines has now been formed.
The formation and development of microcracks in metal occurs as a result of hydrogenation of pipe steel in places of dislocations and vacancies of the crystal lattice and an increase in internal pressure in them to values exceeding the equivalent binding energy of lattice atoms. Hydrogenation itself occurs as a result of the ongoing processes of diffusion of protons (H+), formed as a result of the hydrolysis of water at increased cathodic protection potentials, the dissociation of a number of inorganic compounds, such as bicarbonates, hydrosulfides and sulfides, nitrates, ammonates, phosphates, etc., and the vital activity of sulfate-reducing organisms .
After cracks open on the surface of the pipe in places where the insulating coating of the pipeline is damaged, crack formation accelerates due to the corrosive effect of the soil electrolyte penetrating into the cracks.
The final stage of destruction (including the extent of cracks) is controlled by the conditions of mechanical load on the pipeline, the stress-strain state of the pipe steel, as well as its strength characteristics.
Microbiological corrosion
Microbiological corrosion (or biocorrosion) is metal corrosion that occurs as a result of the activity of microorganisms. Soils and natural surface waters contain a huge number of microorganisms - bacteria, fungi, algae, protozoa, etc. It has now been established that in most cases, metal corrosion is initiated by bacteria due to their high rate of reproduction and activity in chemical transformations of the environment. For the process of microbiological corrosion to occur, the bacteria that cause it must be in a moist or aqueous environment; they also need nitrogen, mineral salts and a number of other elements. It is necessary to have certain external conditions under which they begin to actively multiply near the pipeline, such as:
- temperature;
- pressure;
- illumination;
- hydrogen ion concentration;
- oxygen concentration.
Microorganisms can cause corrosion by producing substances that cause corrosion (for example, acids), creating conditions on the metal surface that cause the appearance of a potential difference on the metal surface and the formation of additional anodic and cathodic zones, with the further occurrence of the corrosion process according to the electrochemical mechanism.
In the case of main pipelines, the most common microbiological corrosion is initiated by sulfate-reducing bacteria. Under the influence of these bacteria, individual cavities form on the pipes. Corrosion products are black in color and smell of hydrogen sulfide. They contain about 40% ferrous iron and 5% sulfur in the form of sulfides. Sulfate-reducing bacteria are present in almost all soils, but significant corrosion occurs only when relatively large numbers of them are present.
So, in this article we briefly outlined the types and mechanisms of corrosion of main pipelines. Read the continuation of the topic dedicated to plumbing systems here .
Cladding is another way to protect metal products from corrosion.
The technology of coating metal parts with a protective anti-corrosion layer of another metal using plastic deformation is called cladding. This processing process is based on cold welding, which allows the formation of atomic bonds between metals without mutual penetration of materials.
Cladding is typically used to form a protective layer or decorative metal coating on parts that are made of stainless steel, structural steel, and copper and aluminum alloys. The processing stages of this technology depend on the method of metal compression and are as follows:
- Preparatory activities, including cleaning the workpiece by mechanical and chemical methods.
- Fixation on the surface of a metal product (sheet, powder or tubular) that is used for cladding.
- Compression of materials, allowing for their deformation, which will be sufficient for their mutual penetration through the formation of atomic bonds.
This technology can be used for processing piece or linear products (sheets, pipes, rods). The procedure for applying a layer that protects against corrosion to metal products can be continuous or cyclic.
In accordance with production needs, cladding technology allows processing up to 6 layers of metal (including the main one). The thickness of the resulting layers ranges from tenths to several millimeters. A necessary condition for the use of cladding technology is the compatibility of the base material with the cladding layer, which is determined by the homogeneity of the crystal lattices.
The most common methods of plastic deformation of metals used to protect metal products from corrosion using cladding technology:
- Rolling. In this case, several layers (from 2 to 4) of long metal are pulled through rollers. Features of multilayer cladding technology are determined by the hardness of the metal sheets and their location in the package.
- Extrusion allows for external and internal cladding of cylindrical products (difficult products, wire, round bars). For this method, a cladding material is used in the form of a tube, which is attached externally to the workpiece. Plastic deformation of materials occurs during the passage of workpieces through a die.
- Stamping. In this case, a sheet of cladding material is applied to the workpiece and pressed, while simultaneously stamping the relief surfaces.
- Explosion welding. Overhead explosive charges are placed above the elements being connected, and when triggered, they are sharply compressed. This technology makes it possible to cladding metal of large thickness.
To ensure the necessary plasticity of the materials being joined, they can be heated using microwave emitters to the desired temperature. The most modern cladding methods include processing using laser equipment. Such devices are equipped with a working body that supplies metal powder directly into the laser beam. The metal is melted and directed in the form of a jet onto the surface of the part.
Consequences of corrosion
Only metals that are found in their pure, native form are not subject to corrosion. For example, gold, silver, platinum. But there are very few of them.
Other metals, under the influence of moisture, return to their natural state in the form of chemical compounds - oxides, salts and hydroxides, from which man extracted them through complex physical and chemical manipulations.
As a result, the environment becomes polluted with heavy metal compounds. They end up in storm drains or streams, and from there into rivers.
After some time, water-insoluble compounds settle to the bottom of urban rivers. If you lift the soil from the bottom, it will not be fertile silt, as in ancient Egypt, but a dead base contaminated with heavy metal compounds. That is why it is useless to drain the reservoirs that were actively built in the USSR during the era of hydroelectric power stations - it will no longer be possible to use the poisoned lands.
But that's not all. Insoluble compounds can undergo chemical reactions and become soluble compounds, which are accumulated by plants.
Both grasses and trees adsorb them, and algae do this even more actively. Metal ions pass through the cell membrane and are absorbed by plants during metabolism. This fact is worth paying attention to anyone who likes to cleanse the body of heavy metals by eating dry algae.
Before feeding on them, you should inquire about the composition of the atmosphere in the place where the algae was collected. So that it does not turn out that the “vacuum cleaner” that is supposed to clean the body is already clogged to the limit.
Reducing the aggressive environment to prevent metal products from corrosion
Modern manufacturing enterprises can successfully use methods for protecting metal products from corrosion based on modifying the composition of the aggressive environment in which metal parts operate. There are 2 ways to reduce the aggressiveness of the environment:
- introduction of inhibitors (substances that slow down the corrosion process of metal products);
- elimination from the working environment of compounds that provoke corrosion processes.
The first method is often used for cooling systems, tanks, pickling baths and other objects with a constant volume of corrosive media.
There are several types of corrosion inhibitors:
- organic, inorganic, volatile;
- anodic, cathodic, combined;
- substances for alkaline, acidic or neutral environments.
The current SNiP requirements allow the use of the following inhibitors:
- Ca(HCO3)2;
- borates and polyphosphates;
- dichromates and chromates;
- nitrites;
- organic substances (polybasic alcohols, thiols, amines, amino alcohols, amino acids with polycarboxyl properties, volatile compounds “IFKHAN-8A”, “VNH-L-20”, “NDA”).
In turn, to reduce the aggressiveness of corrosive environments, the following methods are used:
- vacuuming;
- neutralization of acidic compounds with caustic soda or slaked lime;
- deaeration followed by oxygen removal.
Currently, there is a wide range of technologies for protecting metal parts from corrosion. In each individual case, it is only necessary to select the optimal processing option in order to ensure the highest possible service life for products made of steel and cast iron.