Crushing and grinding
Ore extracted from the depths of the earth is subjected to crushing and grinding, since the size of large pieces during mining exceeds the size of ore pieces allowed under the conditions of blast furnace smelting technology.
For coarse and medium crushing, plants called crushers are used, and for fine grinding, mills are used. Crushing and grinding is an expensive and energy-intensive process. The cost of the ore crushing and grinding process ranges from 35 to 75% of the cost of the entire enrichment cycle. Therefore, it is always advisable to follow the principle of “not crushing anything unnecessary,” that is, crushing the ore only to the required size. To comply with this principle, the crushing process is divided into several stages, using a suitable type of crusher for each stage, and before each of them, classification is carried out in order to isolate ready-sized pieces and fines so as not to subject them to repeated crushing. The following stages of crushing are distinguished:
- coarse crushing from 1500 to 250 mm;
- average crushing from 250 to 50 mm;
- fine crushing from 50 to 5 mm;
- fine grinding up to 0.04 mm.
Crushing is performed using the following methods:
- crushing;
- abrasion;
- splitting;
- blow;
- a combination of the above methods.
For coarse and medium crushing, jaw and cone crushers are mainly used, for fine crushing - roller and hammer crushers, and for fine grinding - ball mills.
Jaw crusher
The jaw crusher consists of three main parts:
- a fixed vertical plate called a fixed cheek;
- movable cheek suspended in the upper part;
- a crank mechanism that imparts oscillatory movements to the movable cheek.
The material is loaded into the crusher from above. When the cheeks come together, the pieces break apart. When the movable jaw moves away, the crushed pieces fall under the influence of their own weight and exit the crusher through the discharge hole.
Cone Crusher
Cone crushers operate on the same principle as jaw crushers, but differ in design.
The cone crusher consists of:
- fixed cone;
- a movable cone suspended at the top;
- drive.
The axis of the movable cone enters eccentrically into the rotating vertical glass, due to which the movable cone makes circular movements inside the large one. When the movable cone approaches some part of the stationary cone, pieces are crushed. And in the diametrically opposite part of the crusher, where the surfaces of the cones are removed to the maximum distance, crushed ore is unloaded.
Roll crusher
In a roller crusher, ore is crushed between two steel rolls rotating towards each other.
Loading is carried out from above, unloading occurs under its own weight. Typically, one roller is stationary, and the second has a special device that allows you to change the gap between the rollers and move them apart in the event of unbreakable pieces of material.
Hammer Crusher
For crushing brittle and clayey ores, hammer crushers are usually used, in which the main part is a rotor rotating at high speed with steel hammers attached to it.
Crushing of the material occurs under the action of numerous hammer blows on falling pieces of material.
Ball mill
For fine grinding, the most common are ball mills, which combine impact with abrasion. They are cylindrical drums rotating around a horizontal axis, in which steel balls are located along with pieces of ore. As a result of the rotation of the drum, the balls, having reached a certain height, roll or fall down, crushing pieces of ore.
The mills operate continuously. Ore is loaded into one hollow axle, and unloaded through another. As a rule, grinding is carried out in an aqueous environment, which eliminates dust emissions and increases the productivity of mills. In addition, particles are automatically sorted by size. Small particles become suspended and are removed from the mill in the form of pulp (a mixture of ore particles and water).
Larger particles that cannot be suspended remain in the mill and are crushed further.
Technological processes of crushing and grinding are almost always combined with sorting and classification of material by size.
The separation or sorting of materials into size classes using mechanical sieves or gratings is called screening , and separation in water or air using the difference in falling speeds of particles of different sizes is called classification . Materials with a particle size of 1–3 mm are usually separated by screening, and smaller ones by classification.
Ore beneficiation
Ore beneficiation is a mineral processing process, the purpose of which is to increase the content of a useful component and reduce the content of harmful impurities by separating the ore mineral from the waste rock. As a result of enrichment, a concentrate , which is richer in the content of a certain metal than the original ore, and a residual product - tailings - which is poorer than the original ore.
The various enrichment methods used in practice are based on the general principle of separating grains of useful minerals and waste rock. The most common methods of beneficiation of iron ores are:
- washing;
- gravity method;
- electromagnetic method;
- flotation.
Flushing
Washing is used for beneficiation of ores with clay and sandy gangue. Typically, rotating drums, so-called butars, having a lattice conical body are used for this purpose. The ore inside the drum moves forward, sliding and rolling along its walls. Under the influence of the pieces hitting each other, the waste rock is destroyed and washed away by jets of water supplied to the drum. The dissolved part of the waste rock, together with water, passes through the holes of the drum, forming waste (tailings), and the washed material (concentrate) is removed through the unloading device.
Gravity method
The gravity method is used when there is a significant difference in the densities of the useful mineral and waste rock.
There are dynamic gravitational enrichment and static enrichment (in heavy suspensions).
Dynamic gravity enrichment
Dynamic gravitational enrichment is based on the difference in the falling speeds of particles of different masses in a liquid. In this case, devices called jigging machines are used, and the enrichment method is jigging.
Crushed ore is loaded onto a grate fixed to the top of a chamber filled with water. The crank mechanism imparts oscillatory movements to the diaphragm, due to which the water level periodically changes. As the diaphragm enters the chamber, a stream of water moves upward through the ore layer on the grate, suspending the ore particles. At the same time, the speed of movement of lighter grains (waste rock) is greater than that of heavier grains (useful mineral). As the flow moves downwards, heavy grains fall faster. As a result of this alternating movement of water flow through the ore layer, it delaminates. In the lower part, closer to the grate, heavy concentrate grains accumulate, and in the surface layer - waste rock grains, which are washed off the grate by the surface layer of water. In recent years, static gravity enrichment (in heavy suspensions) has been increasingly used. The essence of the method is that crushed ore is loaded into a tank with a liquid (suspension) having a density greater than the density of the waste rock, but lower than the density of the ore mineral. In this case, the waste rock floats to the surface of the liquid, and the grains of the useful mineral sink to the bottom of the reservoir. A mixture of water and finely ground ferrosilicon is usually used as a heavy liquid.
Electromagnetic enrichment
Electromagnetic beneficiation is the most common method of beneficiation of iron ores. The method is based on the difference in the magnetic properties of iron-containing minerals and waste rock particles, and consists in the fact that appropriately prepared ore (crushed to a high degree of fineness) is introduced into a magnetic field, under the influence of which grains with magnetic properties are directed in one direction, and non-magnetic ones grains are carried out of the sphere of action of the magnetic field in the other direction.
Magnetic enrichment is carried out in devices called magnetic separators, in which a magnetic field is created by electromagnets. Based on their design, separators are divided into drum, belt, pulley, roller, and ring separators. Drum separators are the most widely used.
Magnetic enrichment of iron ores can be carried out using wet and dry magnetic separation methods. Wet magnetic separation is usually preferred because it eliminates dust formation.
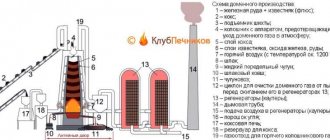
Diagram of a drum electromagnetic separator for ore enrichment in an aquatic environment:
- an electromagnet fixed motionless inside the hollow drum creates a magnetic field on the surface of the left side of the drum;
- magnetic particles of the concentrate are attracted under the influence of this field to the surface of the drum, and then removed from the pulp;
- Using a paper clip and a water nozzle, the concentrate is separated from the surface of the drum outside the magnetic field, and non-magnetic waste rock particles are removed from the separator by a stream of water.
Magnetic separation can, in principle, be used for all iron ore minerals, but effective results can only be achieved with the separation of highly magnetic ores. For weakly magnetic ores, magnetizing roasting is usually used to increase their magnetic susceptibility. Magnetizing roasting is the reduction of iron oxide Fe2O3 into magnetic oxide (magnetite) Fe3O4. Firing is carried out in a reducing atmosphere by burning fuel, using carbon monoxide and hydrogen as a reducing agent.
Flotation
Flotation is used in the beneficiation of oxidized iron ores. The method is based on the distribution of grains of useful mineral and waste rock, which have different wettability with water. The essence of the method is as follows. Air is blown from below into a container filled with water with the addition of special reagents, which rises to the surface in the form of small bubbles. Finely ground ore is continuously poured into the container. In this case, many contacts of air bubbles with ore particles occur. Air bubbles attach to the grains of a poorly wetted (hydrophobic) surface and carry them upward. There is no adhesion between air bubbles and well-wetted (hydrophilic) particles and they sink to the bottom of the container.
Flotation is used mainly for the enrichment of non-ferrous metal ores. In ferrous metallurgy, flotation is used for flotation finishing of iron ore concentrates, as well as for additional extraction of metal from tailings after magnetic and gravitational concentration. For a long time, the use of flotation was hampered by the high cost of flotation reagents, as well as the complexity of wastewater treatment. With the availability of cheap flotation reagents and the improvement of wastewater treatment methods, the use of flotation has expanded.
Extraction of iron ore on an industrial scale
Humanity began to mine ore a very long time ago, but most often it was low-quality raw material with significant sulfur impurities (sedimentary rocks, the so-called “swamp” iron). The scale of development and smelting was constantly increasing. Today, a whole classification of various deposits of ferrous ores has been built.
Main types of industrial deposits
All ore deposits are divided into types depending on the origin of the rock, which in turn makes it possible to distinguish main and secondary iron ore areas.
Main types of industrial iron ore deposits
These include the following deposits:
- Deposits of various types of iron ore (ferruginous quartzites, magnetic iron ore), formed by a metamorphic method, which makes it possible to mine ores that are very rich in composition. Typically, deposits are associated with ancient processes of formation of rocks in the earth's crust and lie on formations called shields.
A crystalline shield is a formation shaped like a large curved lens. It consists of rocks formed during the formation of the earth’s crust 4.5 billion years ago.
The most famous deposits of this type are: Kursk Magnetic Anomaly, Krivoy Rog Basin, Lake Superior (USA/Canada), Hamersley Province in Australia, and the Minas Gerais iron ore region in Brazil.
- Deposits of stratified sedimentary rocks. These deposits were formed due to the sedimentation of iron-rich compounds that are present in minerals destroyed by wind and water. A striking example of iron ore in such deposits is brown iron ore.
The most famous and large deposits are the Lorraine basin in France and the Kerch basin on the peninsula of the same name (Russia).
- Skarn deposits. Usually the ore is of igneous and metamorphic origin, the layers of which, after formation, were displaced at the time of formation of the mountains. That is, iron ore, located in layers at depth, was crushed into folds and moved to the surface during the movement of lithospheric plates. Such deposits are often located in folded areas in the form of layers or pillars of irregular shape. Formed magmatically. Representatives of such deposits: Magnitogorskoye (Ural, Russia), Sarbaiskoye (Kazakhstan), Iron Springs (USA) and others.
- Titanium magnetite ore deposits. Their origin is igneous, most often found on outcrops of ancient bedrock - shields. These include basins and fields in Norway, Canada, Russia (Kachkanarskoye, Kusinskoye).
- About a hundred mineral deposits were discovered in Russia in 2021
Secondary deposits include: apatite-magnetite, magno-magnetite, siderite, ferromanganese deposits developed in Russia, European countries, Cuba and others.
Ore averaging
The composition of ore deposits is in most cases not homogeneous. Areas of rich ore alternate with poorer ore. Therefore, ore mined at one deposit has a variable chemical and mineralogical composition.
Sometimes, fluctuations in iron content in ore reach ± 10%. Fluctuations in the content of the main components of the ore make their further processing difficult. When using non-average iron ores, it is impossible to obtain cast iron of a constant chemical composition, and smelting must be carried out with excessive consumption of coke. At modern ore preparation enterprises, averaging is a mandatory operation, which ensures a deviation in the iron content in the charge within ± 0.3 - 0.5%.
Averaging is the mixing of a large mass of ore material. Typically this operation is carried out in stacks located in homogenization warehouses. The capacity of the stacks can be up to 100 thousand tons. The homogenization warehouse has two stacks, one of which is formed by loading material in parallel layers, usually located horizontally, and the other serves to ship the material for processing. Shipment or collection is also carried out in layers, but in a direction perpendicular to the arrangement of the layers forming the stack. Each portion of material taken, including all forming layers, has a composition equal to the average composition of the material of the entire stack.
Origin of iron ore
All currently known types of ores were formed in three ways:
- Magmatic . Such ores were formed as a result of exposure to high temperatures of magma or ancient volcanic activity, that is, the melting and mixing of other rocks. Such minerals are hard crystalline minerals with a high percentage of iron. Ore deposits of igneous origin are usually associated with old mountain-building zones, where the molten substance came close to the surface.
The process of formation of igneous rocks is as follows: the melt of various minerals (magma) is a very fluid substance, and when cracks form in places of faults, it fills them, cooling and acquiring a crystalline structure. This is how layers with magma frozen in the earth's crust were formed.
- Metamorphic . This is how sedimentary types of minerals are transformed. The process is as follows: when individual sections of the earth's crust move, some of its layers containing the necessary elements fall under the underlying rocks. At depth, they are susceptible to the high temperature and pressure of the upper layers. Over millions of years of such exposure, chemical reactions occur here that transform the composition of the source material and crystallize the substance. Then, during the next movement, the rocks end up closer to the surface.
Typically, iron ore of this origin does not lie too deep and has a high percentage of useful metal composition. For example, a bright example is magnetic iron ore (up to 73-75% iron).
- Sedimentary . The main “workers” in the process of ore formation are water and wind. Destroying rock layers and moving them to lowlands, where they accumulate in the form of layers. Plus, water, as a reagent, can modify the source material (leach). As a result, brown iron ore is formed - crumbly and friable ore containing from 30% to 40% iron, with a large number of various impurities.
Due to various ways of formation, raw materials are often mixed in layers with clays, limestones and igneous rocks. Sometimes deposits of different origins can be mixed in one field. But most often one of the listed breed types predominates.
Having established through geological exploration an approximate picture of the processes occurring in a particular area, possible locations with iron ores are determined. Like, for example, the Kursk magnetic anomaly, or the Krivoy Rog basin, where industrially valuable types of iron ore were formed as a result of magmatic and metamorphic influences.
agglomeration
It is a process of converting small particles of ore concentrates and some other materials into larger pieces (20 - 40 mm) that meet the requirements of blast furnace smelting. There are mainly two methods used for agglomeration:
- agglomeration;
- obtaining pellets.
There is also a third method of agglomeration - briquetting. However, briquetting is not widely used for metallurgical ores due to the difficulty of processing briquettes to obtain the required strength and low tool life.
Agglomeration and production of pellets refer to thermal methods of agglomeration, when a lump product is obtained as a result of sintering and fusion of charge particles heated to high temperatures (1300 - 1500 °C). Due to this, in addition to the physical process of sintering, chemical and mineralogical transformations also occur (decomposition of carbonates, oxidation of sulfur, removal of hydrate moisture, etc.), which improve the quality of the sinter and pellets.
Agglomeration
Agglomeration is the process of agglomeration of small materials (ores, concentrates, flue dust) by sintering as a result of combustion of fuel in a layer of sintered material.
The sintering mixture includes the following components:
- iron-containing materials (concentrate, ore, flue dust) – 40 – 50%;
- flux (limestone), which improves the performance of blast furnaces - 10 - 15%;
- return (small, substandard agglomerate) – 20 – 30%;
- solid fuel (fine coke) – 4 – 6%;
- moisture (added to improve granulation of small particles of the charge) –6 – 9%.
The sintering charge, composed of the indicated components, after mixing and pelletizing, is laid in a layer on the grate of the sintering machine, under which a vacuum is created to maintain the fuel combustion process by sucking atmospheric air through the charge.
The main part of the sintering machine is a kind of metal trough formed from tightly connected trolleys with sides (pa-let) moving along rails on rollers. The bottom of the carts are grates. The movement of the trolleys is carried out along special guides.
The prepared charge is loaded onto continuously moving pallets, which move under the ignition device (forge), where the charge is ignited. After ignition, air is sucked into the layer, ensuring the normal course of the sintering process or moving the agglomerate formation zone downward. The speed of movement of the pallets is adjusted so that the agglomerate formation zone reaches the grate at the moment when the pallet passes over the last vacuum chamber. When the pallet is tipped over, the agglomerate falls under its own weight and, after crushing and screening, is sent for cooling.
Agglomeration should be considered more broadly than agglomeration, since this removes some harmful impurities (sulfur and partially arsenic), decomposes carbonates and produces lump porous fluxed material.
The fuel combustion conditions in this process are very rational. In the combustion zone, the temperature reaches 1500 °C and the combustion products, passing through the charge layer, give off their heat to the lower layers.
Fuel burns to carbon monoxide according to the following reactions:
C + O2 = CO2,
CO2 + C = 2CO.
Iron oxides are reduced by the following reactions:
3Fe2O3 + CO = 2Fe3O4 + CO2,
Fe3O4 + CO = 3FeO + CO2.
The presence of FeO facilitates the production of FeO ⋅ SiO2 (fayalite), which has a relatively low melting point (about 1200 °C), promoting sintering and strengthening of particles.
During agglomeration, sulfur burns out significantly, which is usually found in the charge in the form of iron sulfide FeS2, called pyrite. Pyrite under agglomeration conditions releases sulfur according to the reaction:
3FeS2 + O2 = Fe3O4 + 6 SO2
Limestone decomposes according to the reaction:
CaCO3 → CaO + CO2.
The resulting CaO combines with FeO, SiO2, Fe2O3, forming low-melting compounds with a melting point of 1200 - 1250 °C.
Currently, fluxed agglomerate is mainly obtained. The main advantages of using fluxed agglomerate are:
- exclusion from blast furnace smelting of the carbonate decomposition reaction CaCO3 → CaO + CO2, which requires heat and, consequently, coke consumption;
- improving the reducing ability of gases in a blast furnace due to a decrease in the amount of CO2, since the decomposition of carbonates with the release of CO2 occurs outside the blast furnace, during agglomeration;
- reducing the number of materials loaded into the blast furnace;
- improvement of the slag formation process, since in the fluxed agglomerate the oxides are in close contact with each other.
The use of fluxed agglomerate reduces coke consumption by 6–15%.
Receiving pellets
The process of obtaining pellets has found application in connection with the expanding use of low-grade ores and with the desire for deeper beneficiation associated with the fine grinding of iron ore concentrates.
The most appropriate way to agglomerate finely ground concentrates is to obtain pellets. The technology for the production of iron ore pellets consists of two stages:
- obtaining raw pellets;
- hardening firing.
The composition of the charge for producing pellets includes three main components:
- finely ground ore concentrate;
- bentonite is a special type of clay that increases the plasticity and strength of pellets;
- limestone.
The prepared mixture, after thorough mixing, is sent to granulators, in which, when moistened to 8–10%, pellets of a certain size are formed (balls with a diameter of 10–20 mm).
To ensure strength, the pellets are subjected to hardening firing at a temperature of about 1300 °C. Strengthening of pellets during their firing is achieved as a result of baking small ore particles to each other without the formation of a liquid phase or with a minimum amount of it. During the roasting of pellets, most of the sulfur is removed, limestone is dissociated, and new minerals are formed.
The quality of pellets is characterized by particle size distribution, strength and chemical composition. High-quality pellets must be uniform in size (fraction 10 - 20 mm) and have sufficient strength to withstand transportation, reloading and blast furnace smelting without significant damage.
How to melt metals?
After obtaining resources, building a smelter and a coal furnace, you can get to work and smelt ore into ingots.
First, go to the coal stove and throw wood into it
(maximum 25 pieces) to light the fire and start mining coal.
After some time, coal (charcoal) will start flowing out of the stove
- one piece at a time. Collect it and go to the smelter.
On the right side of the smelter you will find a rectangular hole through which you can throw previously created coal
– up to 20 pieces at a time.
On the other side of the smelter you will find a round hole through which you can throw your chosen ore into the furnace
eg copper ore and/or tin ore - you can mix them, it doesn't matter, they just melt. in the order in which they were added.
After some time, ready-made resource ingots will fall to the ground
, which you can use in the forge and create new items from them.