Compound
Steel is iron enriched with carbon during the smelting process. Carbon smelting is characterized by the presence of carbon, which determines the basic properties of the metal, and impurities: phosphorus (up to 0.07%), silicon (up to 0.35%), sulfur (up to 0.06%), manganese (up to 0.8% ). Thus, low-carbon steel contains no more than 0.25% carbon.
As for other additives, manganese and silicon serve deoxidation (removal of oxygen from the liquid metal, which reduces brittleness during hot deformation). But an increased percentage of sulfur can lead to cracking of the alloy during heat treatment, and phosphorus - during cold treatment.
High-quality engineering steels
In the broad classification of alloys of this type, ordinary quality steels and high-quality engineering (structural) steels are distinguished. The former are not subject to special requirements regarding the choice of charge and melt. The resulting alloy may contain up to 0.08% phosphorus and up to 0.06% sulfur. The material is used for the production of hot-rolled metal.
The requirements for the technology of smelting high-quality steels are higher. Melting is carried out only in electric furnaces, which allow high-precision regulation of the thermal regime and the use of fluxes and slags. The result is an alloy with a minimum content of harmful impurities: phosphorus in it is no more than 0.035%, sulfur - no more than 0.04%.
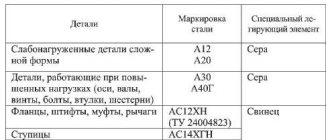
These steels are used in critical equipment components, including gaskets, coils and bushings. In metal marking, numbers from 08 to 25 indicate the mass fraction of carbides - compounds of metals or non-metals with carbon.
Mass fraction of elements in the chemical composition of high-quality low-carbon steels:
- Silicon - 0.17-0.37%
- Manganese - 0.35-0.65% (St 08-20)
- Chromium - >0.1% (St. 08), 0.15% (St. 10), 0.25% (St. 15, 20, 25), 0.50-0.80% (St. 25).
The mass fraction of carbon varies from 0.05-0.12% (St 08) to 0.22%-0.30% (St 25).
Methods of obtaining
The production of a low-carbon alloy can be divided into several stages: loading cast iron and scrap (charge) into the furnace, thermal exposure to a state of melting, and removal of impurities from the mass.
Next, steel casting or additional processing can occur: with slag or vacuum and inert gases.
To carry out such processes, three methods are used:
- Open hearth furnaces. The most common equipment. The melting process takes place over several hours, which allows laboratories to monitor the quality of the resulting composition.
- Convector ovens. Produced by purging with oxygen. It should be noted that alloys obtained in this way are not of high quality, as they contain a larger amount of impurities.
- Induction and electric furnaces. The production process uses slag. In this way, high-quality and specialized alloys are obtained.
Let's consider the features of the classification of alloys.
Low carbon steel production method
The method of producing low-carbon steel refers to ferrous metallurgy, specifically the production of low-carbon steel. The method includes smelting a low-carbon semi-product in a steel-smelting unit, releasing it into a steel-pouring ladle, preliminary deoxidation by supplying ferrosilicon in an amount ensuring the removal of at least half of the oxygen content from the low-carbon semi-product, final deoxidation with an aluminum additive and alloying with manganese during the tapping process to fill the steel-pouring ladle with at least by 0.2 of its height. Alloying with manganese is carried out by supplying a single portion of manganese-containing oxide material together with a slag-forming material in an amount ensuring a basicity of 1.7-1.8. The use of the invention ensures an increase in the degree of absorption of manganese and aluminum while eliminating the use of metallic manganese.
The invention relates to ferrous metallurgy, specifically to the production of low-carbon steel.
There is a known method of steel production, which includes melting steel in a steel-smelting unit, releasing it into a steel-pouring ladle, blowing the metal after release with argon during the flow, introducing aluminum into the ladle with a specific consumption of 2.0 kg/t of metal and metal manganese with a specific consumption of 1.71 kg/t. t of metal (SU No. 1235924 A1, class C21C 7/00, published 06/07/1986).
This method solves the particular problem of reducing the residence time of metal in the ladle and increasing the efficiency of out-of-furnace metal processing. At the same time, the useful use of aluminum for metal deoxidation and residual aluminum in steel is at the level of 50%, and the absorption of metallic manganese by the metal does not exceed 70%.
The closest analogue of the claimed invention in terms of technical essence and achieved result is a method for smelting steel for auto sheets, including smelting the semi-product in a steel-smelting unit, releasing non-deoxidized metal into a ladle, blowing the metal in the ladle with an inert gas to obtain a temperature of 1585-1595°C, preliminary deoxidation with a carbon block with keeping it in the metal at a depth of 20-80% of its height for 2-5 minutes, without stopping the blowing, final deoxidation and alloying with manganese additive of aluminum and metallic manganese in portions weighing 200-750 kg each, while the consumption of metallic manganese is 1- 2.5 kg/t steel, aluminum 1-2.5 kg/t steel (SU No. 981385 A1, class C21C 7/00, published 12/15/1982).
The known method does not achieve the required technical result for the following reasons.
Feeding aluminum and metallic manganese into a 300-ton steel-pouring ladle at costs of 450 and 750 kg, respectively, indicates that the useful use of aluminum for deoxidation and the residual content in the metal does not exceed 40%, and the loss of metallic manganese is more than 30%. In addition, the use of metallic manganese in a known method as a deoxidizer leads not only to its irrational consumption, but also contributes to the deterioration of the quality of the finished metal due to its contamination with manganese oxides, which cannot be reduced by aluminum introduced simultaneously due to the low concentration of oxygen in the resulting non-metallic inclusions. Preliminary deoxidation of the non-deoxidized intermediate product with a carbon block followed by the addition of metallic manganese to it promotes the formation of manganese carbides Mn3C - the most fragile microstructure leading to destruction during stamping.
The basis of the invention is the task of improving the method of steel production by optimizing technological parameters. The expected technical result is an increase in the degree of absorption of manganese and aluminum while eliminating the use of metallic manganese.
The technical result is achieved by the fact that in the method of producing low-carbon steel, including smelting a low-carbon intermediate product in a steel-smelting unit, releasing it into a steel-pouring ladle, preliminary deoxidation, final deoxidation with an aluminum additive and alloying with manganese, according to the invention, preliminary deoxidation is carried out by supplying ferrosilicon in an amount that ensures the removal of less than half the oxygen content of the low-carbon semi-product, and the final deoxidation with an aluminum additive and alloying with manganese is carried out during the production process by filling the steel-pouring ladle to at least 0.2 of its height, while alloying with manganese is carried out by supplying a single portion of manganese-containing oxide material together with a slag-forming amount , ensuring a basicity of 1.7-1.8.
When developing the technology for the production of low-carbon steel with the replacement of metallic manganese with manganese-containing oxide material, it was found that the most acceptable basicity of the slag formed during the reduction of manganese should be 1.4-1.5. Due to the fact that the low-carbon intermediate product produced from the steel-smelting unit contains a large amount of oxygen, a solution was experimentally found by which, under the conditions of the proposed method, a reduction of half of the oxygen contained in the low-carbon intermediate product was ensured. The resulting silicon oxides are adsorbed by the slag, reducing its basicity from 1.7-1.8 to the required 1.4-1.5. Therefore, effective preliminary deoxidation with ferrosilicon ensures the minimization of non-metallic inclusions, and also, due to the absence of carbon entering the metal, prevents the formation of manganese carbides Mn3C, which negatively affect the stamping of rolled products from low-carbon steel.
Since in the proposed method alloying with manganese is carried out by supplying a single portion of manganese-containing oxide material together with a slag-forming material, manganese is introduced into the metal by reducing it from oxides, thereby preventing the formation of manganese oxides, which also negatively affect the quality of the finished steel.
The final deoxidation of aluminum with an additive, which is carried out during the production process simultaneously with alloying with manganese, ensures an increase in the beneficial use of aluminum and manganese. Aluminum in the proposed method is not removed into the gas phase, since the amount of oxygen in the easily reduced manganese oxide contained in the slag is many times greater than the oxygen content in the low-carbon product, especially after preliminary deoxidation. Therefore, in the process of interaction of aluminum with oxygen of the metal and slag, practically no under-oxidized gaseous aluminum oxides are formed, which is the reason for the removal of aluminum into the gas phase. This leads to rational use of aluminum and low losses.
High extraction of manganese is ensured by technological methods that do not involve deoxidation of the metal by manganese, and therefore its additional consumption.
The absence of carbon and silicon in the reduced products in the proposed method ensures the complete elimination of metallic manganese as a deoxidizer and alloying material.
The supply of materials into the steel-pouring ladle by filling it with metal to at least 0.2 of its height is determined by the hydrodynamics of the liquid metal during release. Once the ladle is filled with metal up to 0.2 of its height, intensive mixing of the metal occurs with the formation of vortex-like flows concentrated at the walls of the ladle. Therefore, the supply of materials during this period is accompanied by their intensive mixing with the metal melt, metallization, a decrease in the melting rate and a deterioration in all technological indicators. Once the ladle is filled with metal to at least 0.2 of its height, the surface of the liquid metal comes to a relatively calm state and the danger of the supplied materials being involved in the volume of the metal melt is sharply reduced, which helps to improve technological indicators, in particular, an increase in the extraction of manganese from the oxide material and beneficial use aluminum
Example.
In the production of steel grade 08Yu, a low-carbon intermediate product was smelted in a 160-ton oxygen converter, which at a temperature of 1650°C and the chemical composition, wt.%: C 0.03; Mn 0.05; S 0.015; P 0.008 was released into a steel-pouring ladle. At the beginning of production, ferrosilicon FS-65 was added to the ladle in a single portion with a specific consumption of 0.9 kg/t of low-carbon semi-product. When filling the steel-pouring ladle, manganese-containing oxide material containing 40.0% manganese was supplied to a height of 0.2, with a specific consumption of 6.2 kg/t of low-carbon intermediate product, aluminum with a specific consumption of 1.3 kg/t of low-carbon intermediate product, and lime with a specific consumption 2.5 kg/t of low-carbon intermediate product, providing a basicity of 1.75. The finished metal had the following chemical composition, wt.%: C 0.03; Mn 0.27; S 0.010; P 0.007; Al 0.040. Manganese metal was not used. The degree of manganese extraction was 94.3%, aluminum losses did not exceed 5.0%.
The use of the proposed method for the production of low-carbon steel ensures an increase in the degree of assimilation of manganese and aluminum while eliminating the use of metallic manganese.
A method for producing low-carbon steel, including smelting a low-carbon semi-product in a steel-smelting unit, releasing it into a steel-pouring ladle, preliminary deoxidation, final deoxidation with an aluminum additive, and alloying with manganese, characterized in that preliminary deoxidation is carried out with ferrosilicon, supplied in an amount ensuring the removal of at least half of the content oxygen from the low-carbon intermediate product, and the final deoxidation with an aluminum additive and alloying with manganese is carried out during the tapping process by filling the steel-pouring ladle to at least 0.2 of its height, while alloying with manganese is carried out by supplying a single portion of manganese-containing oxide material together with slag-forming material, the amount of which provides slag basicity equal to 1.7-1.8.
Kinds
Low carbon steel can be of three types:
- Regular quality. In such alloys the sulfur content does not exceed 0.06%, phosphorus 0.07%.
- High quality. Contains: sulfur up to 0.04%, phosphorus up to 0.035%.
- High quality. Sulfur content up to 0.025%, phosphorus up to 0.025%
- Special quality. Low impurity content: sulfur up to 0.015%, phosphorus up to 0.025%.
As mentioned earlier, the fewer impurities, the better the quality of the alloy.
Low-carbon steel GOST 380-94 of ordinary quality is divided into three more groups:
- A. Determined by its mechanical properties. The form of delivery to the consumer is most often found in the form of multi-profile and sheet products.
- B. Main indicators - chemical composition and properties. Optimal for mechanical pressure under thermal factors (forging, stamping).
- B. For these types of alloys, the following properties are important: technical, technological, physical, chemical and, accordingly, composition.
According to the deoxidation process, steel is divided into:
- Calm. The hardening process occurs calmly. Gases are not released during this process. Shrinkage occurs in the middle of the ingot.
- Semi-calm. An intermediate type of steel between calm and boiling compositions.
- Boiling. Solidification occurs with the release of gas. Concealed type shrinkage cavity.
Classification and brands
There are several main criteria by which carbon grades are divided. One of the most important among them is the conditions for deoxidation. The following low-carbon steels are distinguished:
- Calm. Includes a minimal content of iron oxide, which makes the smelting process “calm” - without the violent release of carbon dioxide from the metal surface. This became possible thanks to the introduction of deoxidizing agents: aluminum, manganese and silicon. All escaping gases accumulate in the shrinkage cavity, which is subsequently cut off, resulting in a dense and homogeneous metal.
- Boiling. They are deoxidized by manganese alone. They have an increased amount of iron oxide in their composition. The smelting process is accompanied by the release of carbon dioxide, which gives the impression that the metal is boiling. These steels are less durable and less homogeneous in chemical composition, but at the same time they are cheap and have a low percentage of waste in production.
- Semi-calm. In addition to manganese, aluminum is also used to remove oxygen. According to the characteristics, this carbon steel is something between boiling and calm alloys.
In addition to the degree of deoxidation, low-carbon grades are also classified according to the presence of non-metallic inclusions in their composition. Based on this, they differ in:
- Ordinary quality;
- High-quality mechanical engineering.
Let's look at each point in more detail.
Steel of ordinary quality . They are not subject to strict requirements both for the choice of charge and for melting and casting. Phosphorus in them is allowed no more than 0.08%, and sulfur no more than 0.06%. Such an alloy is poured into large-sized ingots, so they are characterized by the appearance of zonal segregation.
Ordinary quality steel is used for the production of various types of hot-rolled metal: rods GOST 4290-90, channels GOST 8240-97, beams GOST 8239-95, angles GOST 8509-95 and others. This rolled product serves as a material for the production of various types of bolted, riveted and welded metal structures. In the machine tool industry, it is used to produce low-critical parts that do not require heat treatment: axles, rollers, clamps, etc.
Based on the guarantee of the specified properties, ordinary quality steel is:
- Group "A". Delivery occurs according to mechanical characteristics, the chemical composition is not standardized. Marked with “St” and a number from 0 to 6. (Art. 6, Art. 5, etc.). As the number increases, the strength of the selected alloy also increases.
- Group "B". Such metals come with a standardized chemical composition. The marking additionally specifies the method of producing the alloy.
- Group "B". Here, the strength characteristics and chemical composition of steels are controlled simultaneously. The marking additionally indicates the letter B.
High-quality engineering steels are produced under more stringent smelting conditions. They have fewer harmful substances in their chemical composition: sulfur up to 0.04%, phosphorus up to 0.04%. They are marked with the inscription “steel” and a number indicating the amount of carbides in hundredths of a percent.
Steel 08 and 10 are used in critical components of mechanical engineering. They are used to produce bushings, coils, gaskets, etc. Before use, all parts must undergo cementation or any other chemical-thermal hardening.
Steels 15, 20, 25 are used for components that are subject to wear and do not experience increased mechanical loads: levers, gears, valve pushers, etc.
Basic properties
Low-carbon steel is characterized by high ductility and is easily deformed when cold or hot. A distinctive feature of this alloy is its good weldability. Depending on the additional elements, the properties of steel may change.
Most often, low-carbon alloys are used in construction and industry. This is due to the low price and good strength properties. This alloy is also called structural alloy. The properties of low-carbon steel are encrypted in the marking. Below we will look at its features.
Low carbon and low alloy steel
Low-carbon steel for building steel structures is smelted according to GOST 380-60. Depending on the smelting method, steel is divided into open-hearth, oxygen-converter and Bessemer.
According to the deoxidation method, steel is supplied in three modifications: boiling, semi-calm and calm.
Depending on the guaranteed characteristics, steel is divided into three groups: group A, supplied by mechanical properties, group B - by chemical composition and group C - by mechanical properties and chemical composition.
For welded load-bearing structures, steel grade St.3, group B, boiling, semi-quiet or calm grades BM St.3kp, BK St.3kp, BM St.3ps, BK St.3ps and BM St.3sp, BK St.3sp are used. The letters M and K indicate, respectively, the open-hearth or converter production method. For this steel, the guaranteed characteristics according to GOST 380-60 are: yield strength, tensile strength and relative elongation, as well as upper limits for carbon, sulfur and phosphorus content.
At the customer's request, the steel must be provided with:
- satisfactory results for cold bending;
- impact strength at normal temperature {+20° C} for mild and semi-mild steel with a thickness of 10 to 25 mm;
- impact strength at a temperature of -20° C or after mechanical aging for mild and semi-mild steel with a thickness of 10 to 25 mm;
- control chemical analysis of finished rolled products, guaranteeing weldability based on the technological process of steel production, without any special tests.
For bridge construction, steel grades St.3kp bridge, St.3sp bridge and M16S are produced according to GOST 6713-53.
Low-alloy steels are supplied in accordance with GOST 5058-65 and according to special technical conditions, which indicate additional requirements for a given steel grade that are not included in the standard.
Many grades of low-alloy steels can be used in building metal structures, the most common of which are steel grades 14G2, 09G2, 10G2S1, as well as 15GF, 15HSND and 10HSND {naturally alloyed}.
Low-alloy steels have a lower cold-brittleness threshold than low-carbon steels, so they are used for structures operated at temperatures of -40° C and below. These steels must meet the requirements of impact strength at a temperature of -40°C {or -70°C}, and for structures exposed to direct moving or vibration loads, in addition, impact strength after mechanical aging.
Low-alloy steels with higher strength characteristics can be used instead of low-carbon steels in other operating conditions, but they are more expensive than low-carbon steels, so their use must be justified by technical and economic considerations.
Chapter SNiP II-B.3-62 regulates the purpose of various steel grades depending on operational requirements. The issues of choosing steel grades depending on the types of structures and their operating conditions are described in detail in the book by V. N. Zelyatrov and N. P. Melnikov. The authors proposed a classification of steel according to delivery conditions and recommendations for the purpose of steel of these classes for certain types of structures.
Further:
Special cases of vector fields
Harmonic fields
Basic regulatory documents for the design of metal structures
Designer's work on manufacturability of designs
What are KMD drawings and why are they needed? KMD development in Yekaterinburg!
Examples of working drawings of KMD metal structures
Improper integrals over an unbounded domain
Rapid development of mobile communications in the 20th century
KMD design engineer
Drawing of decomposer casing elements
Scalar field, directional derivative, gradient
Is designing metal structures a profitable business?
Manufacturability of structures during installation
Invariant definition of divergence
Ostrogradsky's theorem
Engraving $\Rightarrow$
Marking features
Ordinary low-carbon steel has a letter designation ST and a number. The number should be divided by 100, then the percentage of carbon will be clear. For example, ST15 (carbon 0.15%).
Let's look at the markings and decipher the notation:
- The first letters or their absence indicate belonging to one or another quality group. It can be B or C. If there is no letter, then the alloy belongs to category A.
- St stands for the word "steel".
- The digital designation is an encrypted percentage of carbon content.
- kp, ps – denotes a boiling or semi-quiet alloy. The absence of a designation indicates that the steel is calm (sp).
- The letter designation and the number after it reveal what impurities are included in the composition and their percentage. For example, G – manganese, Y – aluminum, F – vanadium.
For high-quality low-carbon steels, the marking does not include the letter “St”.
Color coding is also used. For example, grade 10 mild steel is white. Special purpose steels may be designated by additional letters. For example, “K” is used in boiler making; OSV - used for the manufacture of carriage axles, etc.
Manufactured products
There are several groups of steel products:
- Sheet steel. Subtypes: thick-sheet (GOST 19903-74), thin-sheet (GOST 19904-74), wide-sheet (GOST 8200-70), strip (GOST 103-76), corrugated (GOST 8568-78)
- Angle profiles. Equal flanges (GOST 8509-93), unequal flanges (GOST 8510-86).
- Channels (GOST 8240-93).
- I-beams. Ordinary I-beams (GOST 8239-89), wide-flange I-beams (GOST 26020-83, STO ASChM 20-93).
- Pipes.
- Profiled flooring.
Secondary profiles are added to this list, which are formed through welding and machining.
Areas of application
The scope of use of low-carbon steel is quite wide and depends on the marking:
- St 0, 1, 3Gsp. Widely used in construction. For example, reinforcing wire made of low carbon steel,
- 05kp, 08, 08kp, 08yu. Good for stamping and cold drawing (high ductility). Used in the automotive industry: body parts, fuel tanks, coils, parts of welded structures.
- 10, 15. Used for parts not subject to high loads. Pipes for boilers, stampings, couplings, bolts, screws.
- 18kp. A typical application is structures that are produced using welding.
- 20, 25. Widely used in the production of fastening materials. Couplings, valve lifters, frames and other parts of agricultural machines.
- 30, 35. Lightly loaded axles, sprockets, gears, etc.
- 40, 45, 50. Parts experiencing medium loads. For example, crankshafts, friction discs.
- 60-85. Parts subject to high loads. These could be rails for railways, wheels for cranes, springs, washers.
As you can see, the product range is extensive - it’s not just low-carbon steel wire. These are also parts of complex mechanisms.
Low alloy and low carbon steel: differences
To improve any characteristics of the alloy, alloying elements are added.
Steels that contain a low amount of carbon (up to a quarter of a percent) and alloying additives (total percentage - up to 4%) are called low-alloy. Such rolled products retain high welding qualities, but at the same time various properties are enhanced. For example, strength, anti-corrosion characteristics and so on. As a rule, both types are used in welded structures that must withstand a temperature range from minus 40 to plus 450 degrees Celsius.
The main properties of low-carbon steels
Low-carbon steel is characterized by low strength with significant toughness and ductility. The alloy is easily processed by hot deformation, cold drawing, and welds well.
An increase in strength characteristics is achieved by carburization - saturation of the surface layers with carbon, after which the surface layers of the alloy are hardened, acquiring the necessary strength. Induction and electric furnaces are used for surface hardening of low-alloy steel. The inner, not enriched, layers remain soft, viscous, and do not lose plasticity due to the unchanged amount of carbon.
Welding Features
Welding low-carbon steels has high performance. The type of welding, electrodes and their thickness are selected based on the following technical data:
- The connection must be firmly fastened.
- There should be no seam defects.
- The chemical composition of the seam must be carried out in accordance with the standards specified in GOST.
- Welded joints must comply with operating conditions (resistance to vibration, mechanical stress, temperature conditions).
Various types of welding can be used, from gas welding to carbon dioxide welding with a consumable electrode. When selecting, take into account the high fusibility of low-carbon and low-alloy alloys.
As for the specific scope of application, low-carbon rolled products are used in construction and mechanical engineering.
The steel grade is selected based on the required output physical and chemical properties. The presence of alloying elements can improve some properties (resistance to corrosion, temperature changes), but also worsen others. Good weldability is another advantage of such alloys.
So, we found out what products made from low-carbon and low-alloy steel are.
Cementation of Low Carbon Steel
Although mild steel is relatively soft, it can be made significantly harder through a process called case hardening. This heat treatment process literally causes the steel to absorb carbon from a carbon-rich solid, liquid or gaseous environment. Typically, carbon is absorbed only by the surface layer of steel. This gives a very hard surface layer to the part, which is useful, for example, for wear resistance. The core of the part remains low-carbon and therefore plastic and viscous. This is very favorable for reliability and resistance to brittle fracture for the part as a whole.