It should be emphasized that the resistance of aluminum and aluminum alloys to normal environmental conditions is very high. The main source of protection against corrosion is a durable, self-healing oxide film, which is always present on aluminum in the surrounding air atmosphere (Figure 1).
Figure 1 – Natural protection of aluminum from corrosion – surface oxide film [4]
Main types of aluminum corrosion
The following main types are typical for aluminum corrosion [4]:
- General corrosion
- Crevice corrosion
- Frettinig corrosion
- Stress Corrosion
- Galvanic corrosion
- Pitting corrosion
- Intergranular corrosion
- Subsurface corrosion
Figure 2 – General corrosion of aluminum: dissolution of the natural oxide film with solutions of strong alkalis and some acids [4]
Figure 3 – Crevice corrosion of aluminum [4]
Figure 4 – Fretting corrosion of aluminum: mutual friction of two aluminum components under rough contact conditions [4]
Figure 5 – Corrosion of aluminum alloys under stress: under certain conditions in Al-Cu, Al-Mg, Al-Zn-Mg alloys [4]
Figure 6 – Galvanic corrosion of an aluminum alloy occurs under conditions of its wet or moist contact with another, more “noble” metal, such as copper [4]
Figure 7 – Pitting corrosion of aluminum under the influence of chloride ions [4]
Figure 8 – Intergranular corrosion and subsurface corrosion [4]
Depending on environmental conditions, loading and functional purpose of the part, any type of corrosion can cause premature failure. In addition, improper use of aluminum parts and products can aggravate corrosion processes.
Materials that cannot be combined when galvanizing
Home Articles Materials that cannot be combined during galvanization
If iron is coated with zinc using the hot-dip method , we obtain so-called galvanized steel. Having entered into a chemical bond with the metal, zinc forms an anti-corrosion coating. Between the pure zinc, which forms the top layer of the coating, and the iron itself, zinc oxide is sandwiched. The percentage of zinc oxide smoothly transitions from zinc to iron.
Galvanic corrosion occurs when different metals come into contact with each other in the presence of an electrolyte. For example, sea water, which penetrates everywhere on a boat, is an excellent electrolyte. Let's consider the electrical potentials of metals that are most often used in small shipbuilding using the galvanic scale presented below.
However, we will not delve into the jungle of chemistry, but for the concept of practical meaning, we will consider the main details:
- Of the two metals in contact with each other, the one on the left will be subject to corrosion.
- Depending on the following condition, a potential difference of 0.1 Volt is considered safe, and a potential difference of 0.2 Volt is considered acceptable.
- Depending on the exposed surface area of the metals, corrosion spreads at different rates. It is better if the fasteners are made of a more noble metal than the product itself, in which case its service life will be longer. Otherwise, it will very soon begin to corrode.
If you use aluminum fasteners, then it is likely that problems will arise; it is best to use Monel rivets, the alloy of which consists of nickel containing copper, iron and manganese.
Using the data in the table below, you can decide for yourself which parts and which metal fasteners are best used for fastening. For example, parts made of stainless steel and aluminum should not be fastened with zinc rivets, and fasteners made of brass are not acceptable for fastening bronze products.
| Fasteners | ||
| Part material | Acceptable | Unacceptable |
| Cink Steel | Galvanized or stainless steel | Brass and bronze |
| Aluminum | Stainless | Galvanized, brass |
| Brass | Brass or bronze | Stainless |
| Bronze | Bronze or stainless steel | Brass |
| Stainless steel | Stainless or monel | Galvanized or brass |
There are alloys that are galvanic couples in themselves. An example of a galvanic couple is brass; when it comes into contact with an electrolyte, one of its phases begins to corrode. This property is called dezincification. A brass object that has undergone such an interaction loses strength and looks very unpleasant.
Steel and galvanized
On board the boat you should not have items made of low-carbon steel that does not have a protective coating, as it is prone to corrosion. If such products are covered with protective material, then their presence in the boat is quite acceptable. This can usually be achieved by applying a layer of zinc, which has 2 advantages. Zinc copes well with corrosion, and if an electrolyte is present, zinc corrodes before steel.
There are several known methods for applying a zinc layer, the fundamental difference between which lies in the thickness of the layer being formed. To obtain a service life acceptable in a marine environment, the thickness of the protective layer should be approximately 100 microns. This result, up to 125 microns, with hot immersion, is achieved by tinning; a result of up to 40 microns is achieved by painting. In this case, electroplating is not used; with it, a coating thickness of only 20 microns can be achieved.
Thus, shiny galvanized fasteners sold in hardware stores can only be useful when building a greenhouse, but not on a boat, where their service life will be short-lived. “Marine” fasteners simply must be tinned.
Copper
When constructing structures made of wood, nails made of copper material with washers are traditionally used. Such nails are an ideal material for fastening fairly flexible structures. They are corrosion resistant, easy to attach and flexible enough to allow elements to move. Such copper ship nails are still found on sale, despite the advent of structures made of glued materials and fiberglass. However, a decrease in the choice of such nails has been noticed, for example, five-six-millimeter washers are no longer available for sale, and therefore canoe builders are forced to rive nails. Nails of non-standard sizes, so necessary when repairing cladding, also disappear from the counters.
Brass
Brass is traditionally used as screws. Bearing in mind the presence of such a problem as dezincification, it is recommended to use fasteners made of brass material only in protected places, for example, interior design or places where our life does not depend on it.
Bronze
Silicon bronze is the standard fastener material. It is used in the manufacture of nails, bolts, and giant screws. Bronze is quite resistant to corrosion and has a long service life (from 30 to 50 years). Thanks to this, regardless of the high cost, bronze fasteners are quite competitive.
| Types of copper alloys and chemical composition | ||||
| Name | Designation | Compound | Application | |
| Brass | Regular brass | CZ108 | Zn 37% | Internal equipment |
| Marine brass | CZ112 | Zn 37%; Sn 1% | Equipment of pre-war boats | |
| High strength brass | CZ114 | Zn 37%; Mn 2%; Al,5%; Fe 1%; Pb 1.5%; Sn 0.8%. | Rigging shackles, propellers, winches | |
| Corrosion resistant brass | CZ132 | Zn 36%; Pb 2.8%; As 0.1% | Water shut-off and pipe fittings | |
| Bronze | Aluminum bronze | CA104 | Al 10%; Ni 5%; Fe 5% | High-strength equipment |
| Phosphor bronze | PB102 | Sn 5%; P 0.2% | Prefabricated and forged equipment | |
| Silicon bronze | CS101 | Si 3%; Mn 1% | Fasteners | |
| Weapon bronze | LG2 | Sn 5%; Pb 5%; Zn 5% | Casting | |
| Aluminum bronze for casting | AB2 | Al 10%; Ni 5%; Fe 3% | Railing and mast equipment | |
| Al - aluminum, As - arsenic, Fe - iron, Mn - manganese, Ni - nickel, P - phosphorus, Pb - lead, Si - silicon, Sn - tin, Zn - zinc. | ||||
It is undesirable and even unacceptable to create galvanic couples from aluminum and aluminum alloys in combination with copper and copper alloys, palladium, silver, nickel, gold, chromium, platinum, tin and rhodium.
Zinc alloys do not combine:
- with silver,
- palladium,
- birth,
- gold,
- copper and copper alloys,
- platinum.
The combination of chromium and nickel with the following metals is unacceptable:
- copper and copper alloys,
- palladium,
- rhodium,
- gold,
- silver
- and platinum.
Unalloyed steel does not combine cadmium, tin and lead:
- with silver,
- birth,
- platinum,
- palladium,
- gold.
Magnesium and aluminum alloys are not allowed:
- with gold
- chrome,
- silver,
- lead,
- platinum,
- palladium,
- copper,
- rhodium
- and with alloyed and unalloyed steel.
Contact us in any convenient way to get advice on any type of galvanic coating service.
Tel: Email Opening hours: Mon-Fri from 900 to 1800
Our specialists will help you with choosing the type and method of galvanic coating.
make an order.
Galvanic corrosion of aluminum
The most common design errors in aluminum structures are related to galvanic corrosion. Galvanic or electrochemical corrosion occurs when two dissimilar metals form an electrical circuit that is completed by a liquid or film electrolyte or corrosive medium. Under these conditions, the potential difference between dissimilar metals creates an electric current passing through the electrolyte, which (current) leads to corrosion primarily of the anode or the less noble metal of this pair.
The essence of galvanic corrosion
When two different metals are in direct contact with an electrically conductive liquid, experience shows that one of them can corrode, that is, become corroded. This is called galvanic corrosion.
The other metal will not corrode; on the contrary, it will be protected from this type of corrosion.
This type of corrosion is different from the types of corrosion that would occur if both of these metals were placed separately in the same liquid. Galvanic corrosion can happen to any metal as soon as two different metals are in contact in an electrically conductive liquid.
Appearance of galvanic corrosion
The appearance of galvanic corrosion is very characteristic. This corrosion does not spread over the entire surface of the product, as is the case with pitting corrosion. Galvanic corrosion is tightly localized in the area of contact between aluminum and another metal. The corrosive effect on aluminum is uniform; it develops deeper in the form of craters, which have a more or less rounded shape [3[.
All aluminum alloys are subject to identical galvanic corrosion [3].
How to protect a structure or assembly from contact corrosion?
If for design reasons it is not possible to avoid unwanted contact of dissimilar metals, then attempts can be made to reduce galvanic corrosion using the following methods:
- painting surfaces in the area of their junction;
- application of compatible metal coatings;
- isolation of the connection from the external environment;
- electrical insulation;
- installation of non-metallic gaskets, inserts, washers in bolted joints.
Practice shows that in cases where the requirements for the admissibility of contacts of different metals are neglected, one has to pay dearly for this. Incorrect arrangement of contact pairs damages fastening units and metal structures and can cost human life.
Only registered users can leave reviews. Please register
The corrosion process of aluminum and aluminum alloys depends on many factors: environmental conditions, as well as the electrochemical and metallurgical properties of the alloy components.
Battery principle
Galvanic corrosion works like a battery, which consists of two electrodes:
- cathode where the reduction reaction occurs
- anode where the oxidation reaction occurs.
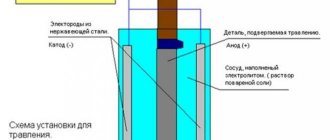
These two electrodes are immersed in a conductive liquid called an electrolyte. An electrolyte is usually a dilute acid solution, such as sulfuric acid, or a saline solution, such as copper sulfate. These two electrodes are connected externally by an electrical circuit that allows electrons to circulate. Inside a liquid, electric current is transmitted by the movement of ions. The liquid thus provides an ionic electrical connection (Figure 9).
Figure 9 – Principle of a galvanic cell [3]
Figure 1 shows a cell in which the electrolyte is a sulfuric acid solution. Sulfuric acid is completely dissociated in water (since it is a strong acid) by forming H+ ions, which determine the acidity of the medium. The following electrochemical reaction occurs [3]:
- The zinc anode is oxidized:
Zn → Zn2+ + 2e−
H+ protons are reduced at the copper cathode:
2Н+ + 2e− → Н2
The complete reaction looks like:
Zn + H2O → Zn(OH)2 + H2
This cell produces electricity by consuming zinc, which is released as zinc hydroxide Zn(OH)2.
For the cell to operate, three conditions must be met simultaneously:
- two different metals that form two electrodes;
- presence of electrolyte;
- continuity of the entire electrical chain.
If any of these conditions are not met, for example if the electrical contact is broken, then the cell will not produce electricity and oxidation at the anode will not occur (nor reduction at the cathode).
Electrochemical corrosion
Previous43Next
Electrochemical corrosion is the destruction of metals in an electrolyte environment.
Parts of many metal structures operate in an electrolyte environment: bridge supports, water transport, steam boilers, chemical equipment, water pipes, car bodies. In damp weather, with daily temperature fluctuations, a film of water condenses on the surface of metals, in which gases from the polluted atmosphere (CO2, SO2, NO2, H2S, etc.) dissolve and an electrolyte is formed.
The surface of metals is heterogeneous. On the surface of the metal there are defects in the crystal lattice, impurities of other metals, inclusions of compounds with non-metals and intermetallic compounds, products of interaction with the environment (oxides, hydroxides, salts, dirt), and surface irregularities. These areas of the metal surface in an electrolyte solution will have a different potential than the base metal. Thus, a system of local galvanic elements short-circuited through the metal is created on the surface of metals. The work of these microscopic elements is accompanied by corrosive destruction of the metal.
As an example, consider the model of an iron-copper galvanic couple in acidic and neutral environments. In a Fe/Cu galvanic couple, iron is more active, the standard potential of which is –0.44 V, and that of copper is +0.34 V. In an acidic environment, an iron oxidation reaction will take place at the anode:
Fe0 –2ē → Fe2+.
Iron ions – Fe2+ – will go into solution, electrons will move to copper, charging it negatively. On the surface of copper, H+ ions from solution are reduced (hydrogen depolarization):
2H+ +2ē → H2.
Total redox reaction of iron corrosion:
Fe0 + 2H+ → Fe2+ + H2.
In neutral and alkaline solutions, at the cathode areas of the metal surface, the process of reduction of oxygen dissolved in water occurs (oxygen depolarization):
O2 + 2H2O +4ē → 4OH–.
Total reaction of the corrosion process:
2Fe0 + O2 + 2H2O → 2Fe2+ + 4OH–, Fe2+ + 2OH– → Fe(OH)2.
As a result of corrosion, Fe(OH)2 is formed, which is further oxidized by atmospheric oxygen to Fe(OH)3, the final oxidation product is hydrated iron (III) oxide (rust):
4Fe(OH)2 + O2 + 2H2O → 4Fe(OH)3 → 4FeOOH + 4H2O.
Rice. 9.4. Scheme of corrosion in an acidic environment upon contact with a less active metal.
Galvanic couples also occur when a metal comes into contact with its chemical compounds. For example, during steel corrosion, the anode is iron grains, and the cathode is iron carbide Fe3C (Fig. 9.5).
Rice. 9.5 .
Scheme of the formation of corrosive galvanic microelements in carbon steel exposed to humid air.
Potential differences can arise due to different concentrations of dissolved oxygen. The solution on the metal surface and in areas where oxygen access is difficult, due to the design features of the parts, contains different amounts of dissolved oxygen, which leads to the emergence of a potential difference in these areas of the parts.
In industrial centers, the atmosphere contains polluting gases: SO2, NO2, CO2, H2S, etc., which, when dissolved in water, produce acids that are aggressive towards metals. In an acidic environment, a corrosion process with hydrogen depolarization will occur on iron. The influence of the composition of atmospheric air on the corrosion rate can be judged from the following data: in rural areas, the corrosion rate of steel is 100-250, in industrial cities 450-550 g/m2 per year.
In a neutral environment (with a fairly clean atmosphere), corrosion occurs with oxygen depolarization. Why is the rate of corrosion with oxygen polarization lower?
In order for corrosion to take place, a necessary condition is that the electrode potential of the metal must be negative than the potential of the oxidizer (depolarizer)
Table 9.1
Dependence of gas electrode potential on pH
| solution pH | E (2H+/H2,Pt), V | Oxidizing metals | E (O2/2OH–), B | Oxidizing metals |
| 0,0 | Pb | 1,23 | Hg | |
| –0,414 | Fe | 0,815 | Ag | |
| –0,828 | Alkaline and alkaline earth, Zn, Al | 0,401 | Cu |
As follows from the data given in the table, most metals can undergo corrosion with oxygen depolarization, but this process is slow, because Oxygen is slightly soluble in water, and the rate of its supply to the metal is low. Almost all of the oxygen arriving at the metal surface is immediately restored. In this case, a protective oxide film may form on the surface of some metals, and some metals, under certain conditions, may even go into a passive state.
Previous43Next
Date added: 2017-11-21; ;
Conditions for galvanic corrosion
Galvanic corrosion is based on the same principle and in order for it to occur, the following three conditions must be simultaneously met [3]:
- various types of metals;
- presence of electrolyte;
- electrical contact between two metals.
Various types of metals
For any metals that belong to various types, galvanic corrosion is possible. The metal with the electronegative potential (or the more electronegative metal if they are both electronegative) acts as the anode.
The tendency of various metals to form galvanic couples and the direction of electrochemical action in various corrosive environments (sea water, tropical climates, industrial atmospheres, etc.) are shown in the so-called galvanic series. The further apart the metals in these rows are, the more severe galvanic corrosion can be. In different corrosive environments, these sequences of metals may be different (Figure 10).
Presence of electrolyte
The contact area must be wetted with an aqueous solution to ensure ionic conductivity. Otherwise there is no possibility of galvanic corrosion.
Electrical contact between metals
Electrical contact between metals can occur either by direct contact between two metals, or through a fastening connection, such as a bolt.
Figure 10 [1]
As can be seen from the graphs in Figure 10, aluminum and its alloys become anodes in galvanic cells with most metals, and aluminum corrodes, as they say, sacrificially and protects the other metal of the galvanic pair from corrosion.
Only magnesium and zinc, including galvanized steel, are more anodic and therefore, being exposed to corrosion themselves, protect aluminum from it.
Aluminum and cadmium generally have almost the same electrode potentials and therefore neither aluminum nor cadmium are subject to galvanic corrosion. Unfortunately, cadmium is recognized as very toxic and is used less and less, and in many countries it is simply banned as an anti-corrosion protection.
Atmospheric corrosion of copper
Under atmospheric conditions, copper is highly resistant to corrosion. In dry air, the surface of copper remains almost unchanged. And upon contact with moist air, an insoluble film is formed, consisting of copper corrosion products such as CuCO3•Cu(OH)2.
Depending on the composition of the environment and many other factors, a very thin protective film consisting of copper oxides and pure copper oxide is first formed on the copper surface in the atmosphere. The formation time of this film can reach several years. The surface darkens a little and becomes brownish. Sometimes the film can be almost black (largely depends on the composition of the corrosive environment). After the formation of the oxide layer, copper salts with a greenish tint begin to accumulate on the surface. The resulting oxide of copper and salt is also called patina. The color of the patina ranges from light brown to black and green. Depends on the quality of surface treatment, the composition of the metal itself and the environment, the time of contact with the corrosive environment (from internal and external factors). Copper oxide is red-brown in color, copper oxide is black. Blue, green, blue and other shades of patina are caused by various copper minerals (sulfates, carbonates, chlorides, etc.). Patina is neutral in relation to the base metal, i.e. does not have a harmful effect on copper (except copper chloride). Salts and oxides that form patina are insoluble in water and have natural decorative and protective properties in relation to the copper surface.
The presence of carbon dioxide in humid air leads to the formation of a mixture on the surface, which is also called malachite. Sulfides and chlorides in the air destroy malachite. This accelerates atmospheric corrosion of copper.
Galvanic couples
The relative position of two metals or alloys in a galvanic series only indicates the possibility of galvanic corrosion if the difference in their galvanic potentials is large enough. This series says nothing more, and especially nothing about the speed or intensity of galvanic corrosion. It may be zero or insignificant or even unnoticeable. Its intensity depends on the types of metals that come into contact - the galvanic couple.
Pair: aluminum – non-alloy steel
In building structures, aluminum parts that are exposed to climatic and weather influences can be connected with screws made of ordinary steel. Experience shows that aluminum in contact with steel screws is subject to only very superficial corrosion. The resulting rust, which has no effect on the aluminum, completely saturates the aluminum oxide layer and forms stains on the surface. In fact, for an aluminum structure in contact with unprotected steel, its effect on appearance and decorative qualities will be more important than its ability to resist corrosion.
This phenomenon has the following explanation:
- On the contact surfaces, films with corrosion products are formed - rust on steel and aluminum oxide on aluminum, which slow down electrochemical reactions.
Pair: aluminum – galvanized steel
Judging by the galvanic series, zinc is more electronegative than aluminum. Fasteners made of galvanized steel can therefore be used for connecting and assembling structures made of aluminum alloys. It must be remembered that when the zinc coating becomes too worn to protect the steel and aluminum, the previous scenario of contact between aluminum and bare steel occurs [3] .
Pair: aluminum – stainless steel
Although there is a large potential difference between stainless steel and aluminum alloys - around 650 mV - it is very rare to see galvanic corrosion on aluminum in contact with stainless steel. Therefore, aluminum structures are very often assembled using stainless steel bolts and screws [3].
Pair: aluminum – copper
Contact between aluminum alloys and copper, as well as copper alloys (bronze, brass) results in very little galvanic corrosion of aluminum when exposed to atmospheric conditions. However, it is recommended to provide electrical insulation between the two metals to contain corrosion of the aluminum.
It should be noted that the product of copper corrosion is the so-called patina. This patina is a bluish-green coating on copper that is composed primarily of copper carbonate. This patina chemically attacks the aluminum and can be reduced to form small copper particles. These copper particles, in turn, can cause localized pitting corrosion of aluminum [3].
Electrochemical corrosion (galvanic couple of copper and aluminum containing mate
Aluminum radiators and copper pipes.
The presence of copper pipes in a heating system based on aluminum radiators does not pose any problems, which can be confirmed based on more than thirty years of experience with installations of this type. In fact, when two different metals or metal alloys come into contact with an electrolyte, one of the two metals may be subject to galvanic corrosion (battery effect).
Electrochemical corrosion occurs only when the following three conditions are simultaneously met: - metals of different nature - the presence of an electrolyte - electrical continuity between two metals The influence of the electrolyte is important if it is characterized by a high salt content, which can occur in a heating system if the chemical and physical characteristics of the water for filling do not meet the characteristics necessary for normal operation of the system, or have been changed since (see the use of salts to control hardness).
Electrical continuity must pass through the valves (brass, steel), connections (steel) and liners (insulating material), which is extremely unlikely if the installation is professionally carried out in accordance with current regulations. In addition, aluminum is a metal subject to natural oxidation, i.e. under natural conditions, a protective oxidized layer forms on its surface, which is also guaranteed by the possible presence of metal particles in the water of the system.
Only thinning of this protective layer can reduce the resistance of the product, a thinning that can occur with aggressive water characteristics. Based on many years of experience gained while working with these systems, we can guarantee the complete safety and compatibility of copper pipes and aluminum radiators. This is taken from Indeed, if in a closed heating circuit with a minor make-up option, a stable water-chemical composition of the coolant with a minimum content of hardness salts (distilled water or ion-exchange softening) is ensured, then this aspect of the problem loses its relevance. And other problems raised at the beginning of the post are solved by various options for mechanical separators at the border of possible contacts. Thank you everyone for your active participation.
How to avoid galvanic corrosion
- Choose a metal pair with aluminum or its alloy that is as close as possible to it in the galvanic series for the corrosive environment in question (see Figure 10).
- Use “cathode” fasteners. Avoid combinations with an unfavorable (large) ratio of cathode to anode areas (Figure 3).
- Provide complete electrical insulation between the two metals being connected. This can be done using insulating spacers, bushings, washers, etc. (Figure 12).
- If painting is used, the cathode should always be painted. If only the anode is painted, any scratch on it will give an unfavorable ratio of the cathode to anode surfaces and will lead to corrosion of the scratch.
- Increase the thickness of the anode or install replaceable massive gaskets made of anode metal into the connection.
- If possible, place the galvanic contact outside the corrosive environment.
- Avoid threaded connections made of metals that form a galvanic couple. Replace them with soldered or welded joints.
- If possible, use corrosion inhibitors, for example in systems with circulating fluid, which can act as an electrolyte for galvanic corrosion.
- In cases where metals must remain in electrical contact through an external electrical circuit, it is necessary to space them as far apart as possible to increase the resistance of the liquid circuit (electrolyte).
- If necessary and where possible, apply cathodic protection with zinc or magnesium sacrificial anodes.
- In the most aggressive environments, only zinc, cadmium and magnesium can be in contact with aluminum without causing galvanic corrosion. Note that the use of cadmium coatings is largely limited due to their environmental unsafety.
Figure 11 [1]
Figure 12 [1]
Sources:
- TALAT 5104.
- Corrosion of Aluminum and Aluminum Alloys. Edited by JR Davis. – ASM International, 1999.
- Corrosion of Aluminum / Christian Vargel – ELSEVIER, 2004
- TALAT 1252