Perhaps the most interesting, based on the variety of qualities, brands, characteristics and applications, is a brass alloy. And, despite the fact that its price is lower than, say, copper, it is used even in the manufacture of jewelry. The composition of brass is simple, but different proportions give such diverse qualities that it needs to be discussed in detail.
Historical reference
According to historical information, the first metals used by man were copper and gold.
Both metals are very soft in their pure state, so their use in human life is quite limited. Copper in particular has been used by ancient people since their early use of fire, and since Roman times the metal has been used more intensively in pipes, military weapons, statue decorations, and other uses. To improve the characteristics of pure metals, for example, greater hardness and strength, over time, man came up with the idea of mixing them. Thus, around 3500 BC, bronze was obtained in Mesopotamia - an alloy of copper and tin, which was highly resistant to corrosion and was stronger than each pure metal separately. Thanks to these properties, bronze began to be used for the production of weapons and tools.
Around 1400 BC, brass was discovered, an alloy of zinc and copper that exhibited excellent resistance to deformation, had high ductility at low and high temperatures, and was highly resistant to corrosion and mechanical wear. However, its use became widespread only in 250 BC with the beginning of coin production in the Roman Empire.
Since that time, the use of brass began to be carried out in a wide variety of areas of human activity, from weapons to jewelry. In the 15th century, it began to be used for the production of astronomical instruments, and with the advent of printing, the alloy began to be actively used in printing. Since the mid-16th century in Europe, bolts and nuts have been made primarily from brass, copper and bronze. This alloy was used to make gears in watch movements, and in the 17th century in Holland, brass was used to make an optical telescope.
Dependence of characteristics on the composition of brass
The properties of brass are directly determined by the mass fractions of the main components. With a zinc content of up to 35%, brass has a single-phase structure (alpha phase), which is characterized by high ductility over a wide temperature range. With a larger proportion of zinc, brass alloys acquire a two-phase structure and become quite brittle under natural temperature conditions.
On sale are two-component grades, consisting only of zinc and copper, and multi-component grades - alloyed with additional chemical elements that modify their properties. Additional alloying components allow you to change individual characteristics, such as strength, toughness, ductility, etc.
- Brass mesh
- Brass square
- Brass sheets L63
- Brass sheets LS59-1
- Brass bands
- Brass rods L63
- Brass wire L63
- Brass pipes L63
- Brass hexagonal rods LS59-1
- Brass hexagonal rods L63
- Brass round bars LS59-1
- Brass wire LS59-1
- Brass pipes L68
Composition and classification of brasses
The classic composition assumes the presence of copper and zinc in the alloy in a ratio of 2:1, respectively. The Ancient Romans knew such brass. Skeptics will remember that zinc in its pure form was discovered in the 16th century. But in the case of Ancient Rome, we are talking about zinc-containing rock, which at that time was already being processed.
At that time, it was believed that it was the presence of zinc that determined the color, and only later did it become known that the sunny shade of the brass alloy is obtained due to the fact that the presence of zinc dilutes the copper redness.
Brass is divided into two-component (simple) and multi-component (special).
One of the markings for products made from brass indicates the percentage of components. So the letter L indicates the type of alloy - brass. and the adjacent numerical index indicates the copper content in the composition. For example, “L80” stands for “brass consisting of 80% copper and 20% zinc.”
Two components are not a mandatory requirement. If there are more of them, then each component introduced into the brass composition is displayed in the marking using the corresponding letter symbol following the letter L. Tin, nickel or lead can be used as additives. At the same time, brass changes its properties.
Additives are introduced into the alloy to achieve certain purposes. For example, brass in classical proportions cannot be used in shipbuilding. All thanks to the instability of brass to the effects of saline solutions (sea water). Additives introduced into the alloy solve this problem while maintaining the basic characteristics.
According to the degree of processing, alloys are divided into: wrought (brass strip, wire, pipe, brass sheet) and cast (fittings, bearings, instrument parts).
Wrought two-component brass
Deformable multi-component brasses
Foundry brasses
Brass alloy production technology
Since the main part of brass is copper, the composition is attributed to copper alloys. The manufacturing method is quite simple. But the technological process itself is complex and labor-intensive, requiring special processing of raw materials and compliance with high temperature conditions.
In the production of brass, technologies from the copper and zinc industries are used. Different types of melting furnaces are adapted for smelting. More often, factories use electrical induction equipment with a magnetic core. An obligatory element is a hood, since when heated, elements dangerous to humans are produced.
Alloy production scheme:
- Melting copper in special crucibles;
- Addition of zinc;
- Introduction of minor components;
- Pouring into molds to produce foundry brass;
- Hardening by stamping or drawing methods.
Physicochemical properties of brass
Brass alloys are marked with the letter LI and numbers that indicate the number of additional chemical elements in the mixture. The characteristics of the copper-zinc composition depend on the ingredients included in the tombak. Therefore, the properties of alloys may differ.
Main properties of brass:
- The color ranges from golden yellow to red depending on the amount of zinc added. Thanks to its beautiful iridescence, tombac is often used to create artistic jewelry.
- Most copper-brass alloys remain ductile when the temperature drops, which makes it possible to use them as a structural material.
- Tompak lends itself well to pressure treatment.
- Possibility of forging and welding.
- High anti-corrosion properties allow brass products to be used for a long time in conditions of high humidity.
- The ability to weld with iron and other components allows the material to be used to produce combined alloys.
- Resistant to wear due to prolonged friction.
- As the temperature decreases, the yield strength and tensile strength of the metal mixture increase.
Methods of obtaining
The brass production process requires the availability of special raw materials - copper, zinc, as well as other metal blanks (lead, aluminum, silicon, nickel), which serve as alloying additives.
The components are placed in a melting furnace with a hood. Copper must first be red-hot, pieces of zinc are added to it, then additives.
Liquid metal is poured into molds to produce casting alloys. If they are subjected to deformation, the output will be deformable brass.
Additives in alloys
Alloying elements are used in brasses. These are substances introduced into the alloy in order to change the structure, and as a result, the characteristics. These elements include:
- Aluminum. The presence of aluminum in the alloy reduces the volatility index. As a result of interaction with oxygen, a layer of aluminum oxide is formed on the surface of the product, which eliminates the volatility of the material.
- Magnesium. This additive is most often introduced in combination with iron and aluminum. Thus, the structure changes, and the alloy becomes stronger, wear-resistant, and corrosion-resistant.
- Nickel. This type of additive is introduced to neutralize the effects of oxidative processes.
- Lead. The presence of this alloying element provides plasticity to the material. It becomes more malleable, easier to mechanically influence, including cutting. Used for products that do not have a load-bearing function during operation.
- Silicon. The additive is introduced to increase the strength of the metal and its rigidity. If lead is added in parallel, the anti-friction properties will improve. Again, alloys of copper, zinc, silicon with lead and bronze with tin are becoming competing. The cost of the latter is higher.
- Tin. This metal is added to eliminate the risk of corrosion pockets. This is especially important in shipbuilding. With the addition of tin, salt water is not harmful to the metal.
Brass production, types and properties
Brass is produced at high temperatures in special clay containers. When making the alloy, it is necessary to take into account that part of the zinc evaporates.
The alloy is divided into several types:
- Tompak is an alloy containing no more than 13% zinc. Tompak is characterized by increased elasticity, high resistance to rust and abrasion. This type of brass is used when welded with stainless steel to obtain a valuable alloy, from which medals, fittings, costume jewelry, art items and tools are subsequently made.
- Semi-tompak is an alloy in which zinc varies between 10-20%. The scope of application of semi-tompak is similar to tombac, but it is a less valuable alloy.
- Cast brass is an alloy containing 50-80% copper, as well as admixtures of other metals. Due to its flowing properties, it is used in the manufacture of semi-finished products and shaped products by casting. It has low rates of material degradation, is resistant to friction and rust and also has excellent mechanical properties. Cast brass is used in the production of bushings, fittings, nuts, bearings and other rust-resistant fittings.
- Automatic brass is an alloy containing lead in a percentage not exceeding 0.8%. Lead allows you to increase the processing speed of products due to the formation of short chips. It is produced in the form of sheets, strips and rods; later they are used to turn watch mechanism parts, hardware and nuts.
This is interesting: Designation and image of the thread in the drawing according to GOST
Quite often, brass is confused with bronze, and many even believe that these are the same material - this is fundamentally wrong. You can distinguish these two metals at home; to do this, you need to go through the following algorithm of actions:
- It is good to clean both materials and examine them in sunlight. The color of bronze will fade to red, and brass to yellow, sometimes even white.
- By placing the product in a container of water, you can analyze it for density. The molar mass of brass is in the range of 8350−8750 kg/m3; if the mass is higher, then it is bronze.
Two-component
The brass alloy, containing mainly only copper and zinc, has only small, trace amounts of other impurities. Pure two-component brass is a phenomenon found only in laboratories. Zinc dissolves in copper at 20-25 degrees by 39%. When heated up to 950°, when the alloy becomes liquid, the solubility of zinc in copper drops to 32%. Attempting to dissolve more zinc at the same 95 degrees will lead to the transition of the brass from the alpha to the beta phase: the excess zinc will either begin to precipitate or remain unevenly suspended, which is why the workpiece cast from beta brass will break at the first serious mechanical (weight) load.
However, the behavior of brass with a gradual increase in the concentration of zinc in the alloy is not quite usual and natural. As long as the zinc content in the alloy is no more than 32%, the ductility of the composition increases. But when passing through 32% at 950 degrees - and during subsequent solidification - fragility and hardness increase. After passing the 45% zinc level, the hardness and strength of the cast workpiece will drop sharply.
Brass can be processed well using high pressure. But at 300-700 degrees the alloy becomes excessively brittle, and in this range brass is not processed in this way.
Cold processing of a two-component alloy is carried out with a zinc content of up to 32%. This is how sheet, wire and profile blanks are obtained. At room temperature, such an alloy is highly plastic. A decrease in ductility at 300-700 degrees does not allow obtaining hot-rolled products - for such, the zinc content would need to be increased to 39%.
The markings for two-component brass are as follows. For example, L-80 is approximately 80% copper and 20% zinc. The number marker indicates the weight percentage of copper in the alloy.
Multicomponent
Grades of multicomponent brass alloys are more numerous than grades of two-component ones. In addition to copper and zinc, alloying is carried out using other components. Simple nomenclature suggests that brass, for example, supplemented with iron and manganese-based impurities is called ferromanganese. Aluminum, for example, has a corresponding name.
Labeling of multicomponent formulations is more complex.
For example, LAZHMts66-6-3-2 contains 66% copper, 6% aluminum, 3% iron and 2% manganese. Zinc is present here in an amount of 23%. Zinc is not indicated in the name: it is calculated by the remainder as a result of subtracting copper and alloying additives. In addition to iron, aluminum and manganese, silicon, lead and nickel are used as additives. Being added in different percentages, they significantly change the properties of the alloy.
- Thus, if manganese is added, the strength and oxidation resistance of brass products increases noticeably. Mixing with tin, aluminum and iron will result in this quality being further enhanced.
- Thanks to tin, not only strength will increase, but also resistance to oxidation in seawater. The fact is that this water contains salts, which under normal conditions would corrode iron and copper even faster than in an environment other than a marine climate. Tin-containing brass is called "marine brass".
- Nickel is distinguished by its ability to form an oxide film on any alloy, which is resistant to destruction. This makes brass less susceptible to corrosion in high humidity environments.
- Lead makes processing easier, but reduces the strength of parts made of brass alloys. The malleability of brass with lead increases significantly. Its content in brass does not exceed 2% - this is how automatic brass is obtained, which received its name due to the fact that the production of parts and components is based on production using automated machines.
- Silicon, although it reduces strength and hardness, in combination with lead it contributes to premature wear of bearing sets.
- Tin – in isolation – due to the anti-oxidation properties of brass in salt water, this alloy can be used in shipbuilding.
Brass exhibits good resistance in solutions of organic acids and salts based on them. The amount and percentage of alloying additives, with the exception of tin, do not have an additional effect on the alloy at this level.
Wrought brass alloys
This category of zinc and copper alloys is pressure processed. The characteristics and technology of working with them are regulated by the GOST standard 15527. They are supplied in the form of rolled metal and blanks for subsequent processing and production of parts of the required shape. Additionally, there are two categories of copper-zinc alloys: double (two-component) and special (multicomponent). The two most popular grades of wrought alloys are L63 (two-component) and LS59-1 (multicomponent, alloyed with lead).
Based on their structure, single-phase and two-phase alloys are also distinguished. Single-phase brass has a uniform, unchangeable color and has good processability. Two-phase ones have increased density, they become more fragile and are less susceptible to cold processing. The melting point for all copper-containing alloys is approximately in the same range.
Alloys, grades
Based on their composition, brass is divided into two-component (zinc alloy) and multi-component. These metal alloys may contain tin Sn, Pb (lead), Al (aluminium), nickel Ni, manganese Mn.
Using the marking formula, it is easy to determine which metals the alloy contains and in what quantities.
The marking of two-component alloys consists of the letter L, to which a number is added - the percentage of copper. Example: L59, L75.
The markings of multi-component brasses indicate the first letters of the metal and its percentage. Example: LTs16K4 - zinc content 16%, silicon 4%, the rest copper.
Microstructure of ground and etched brass alloy under 400x magnification
Brass cannot be found in nature
Informative: native brass was found at the end of the 20th century, it is “zinc copper”.
Brass is practically never found in nature. Deposits of zinc copper are rare and are not of industrial interest. People themselves alloy copper and zinc in the required proportions.
We recommend: URANIUM - a metal for peace and war
The components of the metal alloy are extracted from the corresponding deposits.
Interesting: More than half of the zinc used to produce the gold alloy is obtained through recycling.
What does a ligature give?
| Additive | Property |
| Manganese | Improved corrosion resistance and strength |
| Tin | Increases strength, slows down corrosion in seawater |
| Nickel | Improves overall corrosion resistance in aggressive environments |
| Lead | Improves cutting performance, but degrades mechanical properties |
| Silicon | In combination with lead, it increases anti-friction properties |
The content of more than 20% zinc in the solid melt leads to deformation and corrosion spreading. This drawback is corrected by annealing the products at a temperature of 250-300 degrees.
Important: the more zinc in the alloy, the cheaper it is.
Scope of application of brass
- Jewelry production. Brass is used in the manufacture of rings, bracelets, pendants, commemorative coins, decorative interior items, fittings, and dishes. In this case, different types of brass can be used (more golden, with a greenish tint, classic yellow). Zippo uses brass to make lighter housings. Parker pens also have barrels made from this alloy.
- Mechanical engineering, shipbuilding. Production of parts and components for water transport, aircraft, cars and trucks, as well as specialized equipment.
- Production of fittings. Plumbing equipment (faucets, pipes, fasteners and other components) is made from the alloy.
- Production of spare parts. Brass is used in the manufacture of parts for refrigeration equipment and freezers, which are used in trade and in public catering establishments. The alloy is popular in the production of springs and watch mechanisms. Wire and pipes for various purposes are made from brass. In printing, matrices for fonts are made from such an alloy.
This is interesting: Martensite and martensitic steels: types, structure, transformation
In jewelry
It is usually believed that jewelry should be made of precious metals: gold, silver, platinum. But fashion has its own rules, and for some time now, during the daytime, many women have preferred discreet jewelry. Brass, the color of which is close to gold, is irreplaceable in this case. In addition, it lends itself well to polishing, so with the proper composition and talent of the jeweler, jewelry made from the alloy can look very beautiful and expensive. So that non-specialists will not even suspect that it is not gold, but brass. Photos usually simply do not convey the beauty of skillfully crafted items, so it is better to choose such jewelry in person.
It happens that fashionistas suffer from allergies and irritation. At first glance, it may seem that brass is to blame for everything. But, as a rule, this is not the case. In most cases, the pathological reaction is caused by nickel, which makes the color and overall appearance of the alloy much more beautiful. If you are prone to allergies to metals, it is better to choose jewelry that does not contain this component. Manufacturers usually indicate this separately.
Use in construction and decoration
In construction work, if we mean all finishing engineering work, brass is also used quite often. The most famous area is decorative accessories, since the material is very beautiful in appearance, durable enough for household use and can be easily forged. However, it also has other uses.
Pipes
It is worth immediately noting that in everyday life, in particular for water supply, brass pipes are rarely used. This is due to both the high cost of the material and lower resistance to corrosion compared to products made from pure copper.
The main reason for this choice is the need to achieve a minimum weight of the plumbing system. Such water conduits are made from L-68 brass. They are made by pressing and cold rolling, since the alloy is a two-component alloy, and are well deformed just at low temperatures.
Special-purpose pipes are of much greater importance for the national economy. Their qualities, and, therefore, their application are determined not only by the composition of the alloy - alloying with nickel, manganese, iron, but also by the manufacturing method.
Based on their properties, brasses are divided into casting and wrought. GOST for the manufacture of pipes requires the use of deformable grades in the production of water pipelines. Water conduits are made by pressing, drawing and cold rolling. The pipes do not contain seams, which ensures high mechanical strength.
We consider 2 types of products:
- thick-walled - used in industry, in particular chemical, as they allow the transportation of gas and liquids under pressure;
- thin-walled - used for various types of water supply systems, boiler installations and the like. GOST allows the use of brass pipes for heating installations, but in reality this option is rare.
In addition, not only round pipes are produced, but also square, rectangular and complex shapes. Such products are used in the manufacture of furniture, in structures of a decorative nature, and so on.
We'll talk about kitchen and bath faucets made of brass, couplings and similar products below.
Couplings and mixers
If brass pipes are rarely used in everyday life, then brass fittings make up the bulk of all parts of this kind made of non-ferrous metal. Couplings, tees, nipples, adapters of any kind are used in all engineering systems, including heating ones.
Brass fittings have excellent user characteristics:
- brass parts are much easier and faster to manufacture, since the alloy is perfectly processed by mechanical methods and can be deformed even in a cold state;
- the alloy itself costs less, since zinc is a quite affordable metal;
- Brass is inferior to copper in terms of resistance to corrosion, but this disadvantage is easily compensated for by coating. Chrome or nickel is used for this. Chrome parts are universal: the coating is very hard and has no pores. It is not advisable to combine nickel with ferrous metal, since porosity, although low, is present;
- The strength of brass is, of course, less than that of steel. But unlike the latter, the alloy is much more ductile, so installation and dismantling of systems connected by brass adapters is much easier and faster.
This video will tell you about melting brass and aluminum at home:
Application
Good mechanical properties, relative cheapness - these advantages ensured constant demand for the former orichalcum.
Brass alloys are used in parts for which the following are important:
- plastic;
- deformability;
- fluidity;
- processing ability.
Modern orichalcum has a pleasant golden color. It is used in the production of accessories, artistic products (cups, badges, insignia, orders and medals).
We recommend: PRECIOUS (NOBLE) METALS - rarity, beauty and uniqueness
However, brass is not a precious metal.
- The alloy has found application in the manufacture of pipelines and parts for sea vessels.
- Indispensable in the manufacture of instruments and parts for chemical production.
- Gears, nuts, bushings, bolts - brass is needed everywhere.
- It is used in hydraulic systems of cars, printing matrices, and parts of mechanical watches.
| Double wrought brasses | |
| Brand | Application area |
| L96, L90 | Parts of machines, thermal and chemical equipment, coils, bellows, etc. |
| L85 | Parts of machines, thermal and chemical equipment, coils, bellows, etc. |
| L80 | Parts of machines, thermal and chemical equipment, coils, bellows, etc. |
| L70 | Sleeves for chemical equipment, individual stamped products |
| L68 | Most stamped products |
| L63 | Nuts, bolts, car parts, condenser pipes |
| L60 | Thick-walled pipes, nuts, machine parts. |
| Multi-component wrought brasses | |
| Brand | Application area |
| LA77-2 | Marine condenser pipes |
| LAZ60-1-1 | Details of sea vessels. |
| LAN59-3-2 | Parts of chemical equipment, electrical machines, marine vessels |
| LZhMa59-1-1 | Bearing shells, aircraft and marine parts |
| LN65-5 | Gauge and condenser tubes |
| LMts58- 2 | Nuts, bolts, fittings, machine parts, Soviet small change coin of the 1958 model, in denomination 1-5 kopecks. |
| LMtsA57-3-1 | Details of sea and river vessels |
| LO90-1 | Condenser pipes of heating equipment |
| LO70-1 | Condenser pipes of heating equipment |
| LO62-1 | Condenser pipes of heating equipment |
| LO60-1 | Condenser pipes of heating equipment |
| LS63-3 | Watch parts, bushings |
| LS74-3 | Watch parts, bushings |
| LS64-2 | Printing matrices |
| LS60-1 | Nuts, bolts, gears, bushings |
| LS59-1 | Nuts, bolts, gears, bushings |
| LZhS58-1-1 | Parts made by cutting |
| LK80-3 | Corrosion-resistant machine parts |
| LMsh68-0.05 | Condenser pipes |
| LANKMts75- 2- 2.5- 0.5- 0.5 | Springs, pressure tubes |
Foundry brasses
| Brand | Application area |
| LTs16K4 | Reinforcement parts |
| LTs23A6ZhZMts2 | Solid worm screws, compression screw nuts |
| LCZOAZ | Corrosion-resistant parts |
| LTs40S | Cast fittings, bushings, cages, bearings |
| LTs40MtsZZH | Critical parts operating at temperatures up to 300 °C |
| LTs25S2 | Car hydraulic system fittings |
Jewelry alloys
A brass dice, next to it are zinc and a copper ingot.
| Jewelry alloys | ||
| Type of processing | Color | Alloy name |
| casting | yellow | Brass granules M67/33 |
| casting | green | Brass granules M60/40 |
| casting | gold | Brass granules M75/25 |
| casting | yellow | Brass granules M90 |
We recommend: TITANIUM - the superman of metals
Analogs
Since brass is an alloy of copper and zinc, and there is more of the former, it may seem that pure metals (each separately) have better properties, and such complexities are used to reduce the cost of the material. In fact, everything is not like that. Copper in its pure form has such disadvantages as instability to corrosion, lower ductility compared to alloys, and zinc is extremely fragile. Brass organically combines the best properties, mutually compensating for the shortcomings of both components.
Other copper alloys - bronze, cupronickel, etc. - also cannot be fully called analogues. The first is less plastic and more coarse-grained, while the second substance is quite refractory and, due to the nickel content, can cause skin irritation. In addition, external characteristics also put brass first. The color, similar to gold, compares favorably with the not very attractive brown bronze and silver cupronickel.
World market
Industrial production of brass began almost immediately after its rediscovery. Having assessed its unique properties, metallurgists began to develop a new direction in the industry. Today, the production and consumption of brass mainly depends on the state of the global copper market. Its stable growth gives reason to believe that the demand for alloys is not falling yet. Moreover, forecasts for the future of these industries are more than favorable, despite problems such as declining ore quality, insufficient infrastructure development, and social and political tensions in the largest copper suppliers - Chile and some African countries.
The main consumers of copper, and therefore brass, are the economically developed countries of Europe, as well as the USA, China, Japan and some others. In recent years, the demand for these substances has only been growing, primarily due to Asians. Having made a giant leap in the mid-2000s, Cu prices remain at their previous record highs. However, a peak in supply is expected in 2021, which is likely to trigger a decline in prices.
This is interesting: Metal cutters for a milling machine. What are the types and their uses?
How does bronze differ from brass in composition?
Characteristic differences between these two metals should only be expected if they are fairly pure. But the fact is that now there are a huge number of varieties of both brass and bronze. Very often, tin is not added to bronze at all and a mixture of aluminum, beryllium and magnesium is introduced as an alloying element. Thanks to this, the color of the metal also changes greatly. If the tin content in the metal is high enough, reaching 40%, then its color can fluctuate up to white. That is, it resembles steel.
At the same time, it gives only a light golden tint. In general, the metal turns out almost silvery. Regarding brass, if it contains a large amount of zinc, then the color of the metal is the same as gold. Quite often this material is used to make a variety of costume jewelry and cheap jewelry. Such jewelry looks quite organic, nice, and at the same time has a low price.
Fitting
How to distinguish brass from bronze with a magnet
There is no limit to human misconceptions. Most ordinary people are confident that a magnet is able to give a definite answer. To verify whether this is so, let us return to the chemical composition of the alloys. Of the main components of the compounds: copper, tin, zinc, aluminum, iron and nickel, only the last pair is magnetic. As a result, only grades of alloys containing Fe and Ni are capable of being attracted to a magnet. These are BrAZh bronzes, for example.
The alloy BrAZhN-10-4-4 has the greatest magnetic susceptibility, where the total proportion of iron and nickel is 7 – 11%. However, to get a noticeable effect you will need a powerful magnet, for example neodymium. Among brasses, grades containing iron or nickel are LAZH and LAN, respectively. The proportion of magnetic metals in them is 1–3%, which complicates identification even with neodymium.
However, the weak magnetic properties of some grades of copper alloys lead to rumors that this is an effective way to distinguish brass from bronze.
So, you should know that you CANNOT distinguish brass from bronze with a MAGNET!
Video - Brass and its magnetic properties:
Visual approach
Alloys with a high content of the main alloying component are easily recognizable by color. The technique for visually distinguishing brass from bronze is as follows:
- Brass is an alloy with a high zinc content. This causes the color of the compound to shift from the pink-red hue of pure copper to golden yellow tones. We can confidently say that the color of brass is closer to gold. Although scrap brass comes in different forms and different states, and it is certainly not easy to determine with your eyes, the same applies to scrap bronze.
- Bronze. The quantitative content of tin in the alloy determines the color of the compound. Bronze with a maximum Sn content of 33% is characterized by a silvery-white color. The alloy, containing at least 90% copper, also borrows its color - closer to brown-red tones.
Since in practice, compounds with a high tin content are rare, you can trust the following rule. Brass is a golden-yellow hue, bronze is reddish.
Pure physics
The density of copper alloys is the next criterion for distinguishing brass from bronze. However, the popular belief that scales will give a definite answer is incorrect. Confirmation of this is provided by connection densities:
- rolled brass – 8.4 – 8.7;
- yellow brass – 8.43;
- bronze – 7.4 – 8.9.
All values are given in g/cc. As you can see, the weight of bronze, like its color, is highly dependent on the tin content. When its inclusion is at the level of 8%, the connection density is minimal and lower than that of brass. An increase in tin content leads to a heavier alloy. The result is that such bronze weighs more than brass. Therefore, using mass as a distinguishing criterion for copper alloys is not recommended in practice.
This video outlines the principle of calculating and determining metal based on weight and density:
Heat treatment
Temperature 600-650 °C is critical for zinc. The metal oxidizes when heated this way. This is a real way to visually distinguish bronze from brass in a burner flame:
- Bronze. The alloy will simply heat up. Its color and mechanical properties will remain unchanged. Attempting to bend a bronze specimen may result in its destruction.
- Brass. Oxidation of zinc causes an ashy coating on the surface of the joint. Additionally, after heat treatment at 600 °C, brass becomes ductile, and the alloy sample does not break when bent.
All that remains is to find a powerful burner. Here a gas stove or a lighter flame will not be enough.
Video - Melting bronze and brass:
Welding machine
What is the best way to distinguish bronze from brass? It is necessary to catch the arc with an electrode at the edge of the blank. Bronze has a smokeless process. On the contrary, exposing a brass blank to a welding arc will cause the zinc to burn out. The process is accompanied by the appearance of white smoke.
Chemical technique
The use of reagents is an effective but destructive way to distinguish between copper alloys. Chemical analysis takes place in several stages:
- The shavings are removed from brass and bronze.
- An aqueous nitric acid solution is prepared with a 1:1 ratio.
- The shavings are placed in various containers filled with an acid reagent.
- Each tank is heated to a boil after the chips have completely dissolved.
- The compositions are kept in a boiling state over low heat for 30 minutes.
The result is that the container with brass remains transparent, while a white tin precipitate forms in the bronze container. Naturally, the technology is not suitable for tin-free alloys.
What is brass
The main components of brass alloy are copper and zinc. The proportional components of these metals may be different. The amount of zinc varies. Its minimum value is 20%. The maximum reaches 50%. At the same time, the alloy changes its color: it can be golden, yellow or green.
The percentage of zinc is so important that it can change the characteristics of the material. This refers to its ductility and hardness.
Structure and composition
The composition of the alloy is formed from the phases:
- Alpha phase. Zinc content up to 35%
- Beta phase. The presence of zinc is up to 50%. The composition also includes tin - 6%.
In some cases, a single alpha phase is present. Depending on changes in the percentage composition of the main components, the structure of brass can simultaneously consist of 2 phases - alpha and beta.
The chemical composition of brass, in addition to copper and the main alloying element zinc, includes additives. These include alloying elements: aluminum, iron, manganese, lead, silicon, nickel. They make up a small percentage of the compound. Each of them affects the characteristics of the material.
Properties and characteristics
The main quality in the characteristics of brass is its corrosion resistance. But it also has other properties:
- The ability of the alloy to withstand aggressive environments, especially after coating the surface with varnish.
- Strength of brass.
- Plasticity of the alloy.
- The ability of the material to be processed by pressure. The process is carried out both hot at high temperatures and cold.
- The alloy can be subjected to resistance welding and soldering.
- Thermal conductivity, which increases with increasing percentage of copper.
- Melting point, which is 880–950 degrees. With less zinc added, the melting point decreases.
- The material has non-magnetic properties.
The main factor in the hardness and ductility of the joint is zinc. An increase in its quantitative content is directly related to an increase in strength characteristics. Plasticity increases only up to a quantitative zinc content of 36%. With a subsequent increase to 45%, this indicator decreases.
In order to increase the hardness of the alloy, a heat treatment called cold hardening is carried out. It helps not only to increase the strength index, but also relieves internal, structural stresses.
Alloying additives affect the performance characteristics. Their influence is indicated in the table:
| Name of alloying element | Effect on brass characteristics |
| Silicon | Its high presence leads to a decrease in the hardness of brass. |
| Lead | Improves anti-friction properties. |
| Manganese, aluminum and tin | Increases resistance to tearing. Corrosion resistance is increasing. |
| Nickel | Reduces the risk of material cracking. The alloy acquires a peculiar color. This connection is called “white brass”. |
| Arsenic | The material has the ability to work in liquid, fresh media. |
Marking
There are 2 types of alloys:
- Two-component. The main components are copper and zinc. They are marked with the letter L. Next are numbers indicating the amount of copper in percent. L60: contains 60% copper, and the remaining 40% zinc.
- Multicomponent. In addition to the main components, alloying elements are added. Also in front is the letter L. Then follows a list of additives. At the end, numbers are written through a dash indicating the percentage of each component. The amount of zinc is not indicated, but calculated. For example: Brand LAZhMts66-6-3-2 has 66% Cu, 6% Al, 3% Fe and 2% Mn. By calculation, the amount of zinc is determined to be 23%.
Advantages and disadvantages
Brass alloy has characteristics that are positive in one case and negative in another. They consist of the following:
- Light weight. This quality, together with high strength, is used in certain industries.
- The alloy has good ductility.
- Low cost.
- Corrosion resistance decreases with increasing amount of copper.
- Thermal conductivity indicators are lower than those of pure copper and bronze.
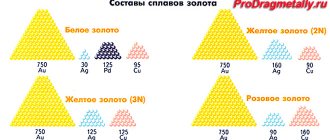
How to distinguish gold from brass
Even though gold and brass look similar, there are ways to tell one from the other. This is checked as follows:
- Gold has a more saturated color. In addition, over time, brass darkens because it oxidizes in air, but gold does not.
- If you put a magnet near it, brass will be attracted, but gold will not.
- Brass has a higher density, which means it is heavier. This is noticeable when throwing pieces of metal in your palms.
- Availability of sample.
- If you test with acid, the gold will not react and the brass will discolor.