A LITTLE ABOUT BRASS
Brass is an alloy of copper and zinc. It is the percentage of the latter that is the determining factor in the presence of certain properties and qualities. The average level of zinc presence is considered to be from 20 to 45%.
As a material, brass is different:
- Ease of processing under pressure;
- Good indicators of anti-corrosion resistance;
- Increasing ductility at low temperatures, while strength does not decrease;
- Increased fragility when exposed to temperatures from 200 to 600 degrees;
- Good anti-friction properties;
- Good ability to weld with other metals.
Brass is one of the most popular alloys both in production and in everyday life. In this regard, questions about caring for alloy objects and how to clean brass and how to clean brass at home .
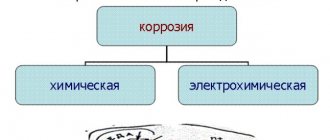
TO
Corrosion of brass in an atmosphere not contaminated with aggressive gases and other impurities (rural areas) is insignificant and increases sharply in the presence of impurities of sulfur dioxide, ammonia, chlorine, and nitrogen oxides in the air (the atmosphere of industrial cities). In sea water, the corrosion depth of brass is 0.04–0.06 mm/year. The most aggressive media for brass are oxidation solutions. to-t, ammonia, mercury salts, copper, ferric iron. Typical types of brass corrosion are: dezincification and cracking. Dezincification is most often observed when brass is exposed to solutions containing chlorine ion. Brasses containing less than 20% zinc are not subject to dezincification. Alloying brass with a few hundredths of arsenic prevents dezincification. Corrosion cracking of brass is called. earlier by “seasonal” cracking. This outdated name arose due to the observed benefits. cracking of brass in higher seasons. humidity (in autumn and spring for the climatic conditions of Moscow, in winter for England).Previously, the term “spontaneous cracking” was also used.
Brass is very prone to corrosion cracking in the presence of tensile (residual or externally applied) stresses in the surface layers and exposure to certain specific conditions. corrosive substances (ammonia, sulfur dioxide, nitrogen oxides, cyanides) or metals that dissolve zinc (mercury, fusible solders). Ammonia (in the obligatory presence of oxygen and moisture) is the most corrosive agent, however, the presence of acidic oxides (even CO2) significantly inhibits the corrosion process and can neutralize the effect of ammonia. The ammonia content in the air is usually extremely low, and the acidic components (for example, sulfur dioxide) are much higher, so practically cracking of brass occurs from exposure to S02, not NH3 (except for places where large quantities of NH3 are released - ammonia plants, stables, bathrooms).
The greater the magnitude of tensile stresses in the surface layers of the alloy, the greater the danger of cracking. Therefore, operations such as mandrel-free drawing of pipes, rolling caps, and assembling brass parts with tension should be avoided.
The most common tests are in an ammonia atmosphere (over aqueous solutions of ammonia of 5,10, 20 or 25% concentration) and in aqueous solutions of mercury salts (nitrate or chloride). The ammonia test is very stringent; in this environment, brass cracks even at very low stresses (1 kg!mm2) or with a low zinc content (3-5%). The mercury test causes cracking of brass at tensile stresses of at least 10 kg/mm2. Testing in an air atmosphere over dilute solutions of sulfur dioxide is more reasonable, since sulfur dioxide is the main corrosive impurity in the atmosphere of populated areas.
Lit.: Tomashov N.D., Theory of corrosion and protection of metals, M., 1959; Corrosion of metals, collection. Art., trans. from English, book. 1-2, JI.-M., 1952; Bobylev A.V., Corrosion cracking of brass, M., 1956.
BRASS. Brass is an alloy of copper and zinc
copper and zinc Brass
| Brass . |
Copper, brass, bronze. Green mordant for copper, brass and...
| Arc welding brass complicated by the fact that when it is heated and melted it evaporates... Bronzes are alloys of copper |
BRASS. BRASS PRODUCTS. Golden-red mordant for brass...
| Non-ferrous metals - brass tin zinc lead aluminum gold bronze |
BRASS. Golden yellow mordant for brass items
| Non-ferrous metals - brass tin zinc lead aluminum gold bronze |
BRASS. Purple mordant for brass items. Antimony oil
| Small brass things, such as buttons, clasps, buckles, etc., can |
Recommended background color for processing non-ferrous parts: copper, brass...
| Recommended background color for processing non-ferrous parts (copper, brass bronze) - light blue or gray-blue; |
Brasses of medieval Novgorod - N. V. Eniosova, R. A. Mitoyan, T ...
| Brass - one of the most unusual alloys of antiquity - appeared in the arsenal of metallurgists much later than all other known metals and alloys. |
Non-ferrous metals and alloys. Nickel and nickel alloys. Titanium alloys
| Brass in pipeline fittings they are used for the manufacture of sealing rings for water, running nuts, and electrically conductive parts of drives. |
PREPARATION FOR CLEANING BRASS
Before you begin cleaning brass, the material must be prepared. Preparation for cleaning brass at home consists of the following activities:
- Determining whether an alloy belongs to brass. Namely, whether the material is pure brass or has impurities. To do this, they usually use a magnet, which attracts the brass;
- The feasibility and safety of the upcoming brass cleaning is assessed. It is usually not recommended to clean antiques yourself due to the risk of damaging the patina layer that performs a protective function;
- Immediately before starting the process of cleaning brass, the product is immersed in a soap solution for 20-30 minutes. After completion of the exposure, large contaminants are removed using a medium-hard brush;
- Products that have a paint coating are washed with soapy water and wiped with a soft cloth;
- Areas with serious (complex) contamination are treated with products that do not contain abrasives;
- The use of acetic and hydrochloric acids is justified only in exceptional cases, due to the risk of damage to the alloy surface;
- Washing dishes made of brass is highly undesirable due to the risk of loss of aesthetic appeal.
Most often, to clean brass at home, they use folk remedies that are available in every home and are always at hand.
How and with what to clean brass
To clean brass furniture at home, you will need only 5 minutes of free time, 3M Marine Metal Restorer and polish 09019e brass cleaning paste, a rag and a dry cloth.
- Step 1: Use masking tape to isolate the brass from contact with other items so that the cleaner does not come into contact with them.
- Step 2: Put on gloves and prepare a rag and 3M Marine Metal Restorer and polish 09019e.
- Step 3: Using a rag, apply a small amount of paste to the item you want to clean. There should be enough paste to cover the entire product with a thin layer. For uniformity, you need to clean the entire surface at once. Check out our Instagram to see how to do it right.
- Step 4. Rub the paste over the product with smooth movements for a couple of minutes. During the cleaning process, the paste will begin to darken. Don't be alarmed, this is a standard chemical reaction.
- Step 5. Use a dry, clean cloth to wipe off any remaining paste. Do not wash off with water!
Ready! The brass looks like new with no finger marks or water marks. Using 3M Marine Metal Restorer and polish paste 09019e, you can clean brass to a shine without harming the product itself.
Brass sconce SB013. Project by designer Natalia Patrusheva
How not to clean brass
The following are not suitable for cleaning brass at home:
- Products with abrasive particles. They may scratch the product.
- Hard brushes. Cleaning should be done with a cloth or soft bristle brush.
- Products containing alcohol.
You can clean brass with citric acid, but only very carefully. In some cases, acid can harm the metal. If you don’t know how the product will behave and what particles are added to the alloy, then it’s better not to take risks. The same goes for acetic and hydrochloric acid.
Chandelier SL023. Designer Natela Mankaeva.
HOW TO CLEAN BRASS AT HOME WITH OXALIC ACID
Acid is used both for cleaning brass from oxide, and for the usual processing of things that have lost their aesthetics. In domestic conditions, oxalic acid is most often found as one of the components of complex care products for decorative items and other accessories.
Treatment of brass with oxalic acid concentrate:
- Wear personal protective equipment (goggles, gloves, apron);
- In a container of sufficient volume, the acid is diluted at the rate of 25 grams of the substance per 1 liter of water;
- The surface of the product is treated with a soft sponge soaked in the solution;
- The applied composition is left on the surface for 15 minutes;
- After completion of the aging period, the brass product is thoroughly washed in a soap solution at 30-40 degrees.
To remove significant oxide deposits, you can use the acid in its pure form without diluting it in water. In this case, the time the acid remains on the surface should not exceed 5 minutes. It is worth considering that it is better not to subject antique products to such treatment due to the excessive aggressiveness of the product.
Cleaning brass with a mixture of acid and soda:
- Technical oxalic acid is applied to the surface to be treated. Contact time is approximately 5 minutes. At this stage the surface darkens, which is a natural reaction;
- Using a medium-hard brush, apply a small amount of soda to the surface. The composition is left for 5 minutes. Particular attention is paid to areas with the most pronounced oxide stains;
- The product is cleaned with a brush and washed under running water.
Professional acid-based brass cleaning products.
Metalin. A liquid containing up to 20% hydrochloric acid and a significant admixture of inhibitors that create a protective anti-corrosion layer. To improve results, you can use Metalin cotton wool.
The composition of Metalin cotton wool includes:
- Olex-2 paste. Bronze Cleaner. A strongly acidic paste-like product that has powerful antioxidant properties due to the creation of a film on the surface. The paste cleans well almost any stubborn dirt, even stubborn dirt. Before use, shake the container with paste thoroughly. The composition is applied to the surface of the alloy and left on it for a period of 5 to 10 minutes. After removing the paste, the product is polished.
- Pasta "Ideal". It is another fairly popular acid-containing brass cleaner.
You can prepare a composition similar in components for cleaning brass at home:
- Melt a mixture of cerisin and wax in a metal container;
- Oleic acid, white spirit and carboxymethylcellulose pre-soaked in water are introduced into the resulting mixture;
- The mixture is heated to 70-75 degrees, stirring constantly;
- Without stopping stirring, triethanolamine, OP-7 and turpentine are added;
- Heating continues for 10 minutes;
- Pumice powder is mixed in small portions;
- The composition is left for 10-15 minutes with constant stirring;
- When the temperature reaches 35-40 degrees, the mixture is poured into a container with a tight-fitting lid.
Taking into account a number of nuances that must be observed during the process and some of its labor intensity, for the most part consumers prefer ready-made pasta. Ideal paste is suitable for treating metal surfaces, including those with an enamel coating, from any type of contamination. It is worth considering that this paste is not recommended for cleaning food utensils.
HOW TO CLEAN BRASS AT HOME WITH ACETONE
It is used both in pure form and as part of nail polish removers. In the question: “ how and with what to clean brass at home ” it is ideal for cleaning both uncoated surfaces and previously painted ones.
Treatment of brass with acetone is carried out in several stages:
- The product is wiped with a clean swab;
- Using a swab, acetone is applied to the surface;
- If there is significant contamination, the process adds additional steps in the form of 3-4 hours of boiling the alloy in a solution of water, salt and vinegar.
It must be taken into account that acetone is an aggressive agent and requires protective equipment (goggles, gloves).
Methods and means for cleaning brass
In order to give the product its original appearance, you need to know how and with what to clean brass at home. When choosing a purchased cleaning product, be sure to pay attention to the composition and acids contained in it. Each of the acids interacts with metals differently, so the probability of destroying the protective layer along with oxidation is quite high.
Read also: The principle of operation of a beer cooler
What is not recommended to use
To avoid damage and destruction of the protective coating, you need to know about the products that are not recommended for use when cleaning brass. Therefore, first we list the substances that are dangerous to brass:
- Vinegar or acetic acid. When interacting with this acid, products made from “eternal” metal undergo dezincification and acquire a bright red color.
- Sandpaper. Even with the smallest abrasive size, sandpaper can not only scratch the item, but also remove part of the protective coating.
You need to be very careful with store-bought chemicals. Pre-cleaning is, of course, necessary. But before you start the process , you must be familiar with the components of the product. Strong chemicals can not only corrode and destroy the top layer, but also change its structure. Watch the brass and do not leave it in a chemical solution for a long time if you decide to clean it using chemicals.
Compositions that will not harm the surface
Before any cleaning, you should verify the composition of your product. Brass may be a metal, but it does not react to a magnet. Therefore, if your product is magnetic, then you should conclude that the composition contains impurities. This means that the cleaning method should be selected carefully to protect the product from damage . If you are completely sure of the composition of the product, then cleaning can be done using the following means:
- Oxalic (ethanedioic) acid or cleaning agents or detergents that contain it. In its pure form, oxalic acid requires 200 g per 10 liters of water. For such a solution, plastic containers are best suited, since metal containers can be exposed to acid. There are two ways to prepare this solution: in cold or hot water. The “cold” method involves completely immersing the product in a solution and periodically monitoring the cleaning process, since this method can take several days. The “hot” cleaning method involves using hot water for the solution. The temperature of the water that flows from the tap will be quite sufficient. Using higher temperature water increases the risk of damage to the paint or the top protective layer, if any. The product should be completely immersed in the solution, otherwise the edges located above the level of the solution will begin to oxidize very quickly due to the combination of acid vapors with oxygen. It is also necessary to maintain the temperature by placing a plastic container with the solution in a hot bath. The time for the first procedure is 20-40 minutes; if necessary, the process can be repeated, reducing the time the product remains in the solution. The prepared solution for subsequent procedures can be stored in plastic containers: bottles or buckets with lids.
- Acetone. A cotton swab is moistened with acetone and wiped over the entire surface of the product. But this method is not suitable for varnished brass products.
- Formic acid. Cleaning is less effective, but possible. 30% acid is sufficient for cleaning. The effect is lower due to the rapid weathering of the component, but, on the other hand, it is gentle and ensures the safety of the product.
- Salt. Ancient cleaning method: 1 tbsp. l. for 1 glass of whey.
- Solutions of ammonia and ammonium carbonate. 10−15% is enough.
- Lemon juice with salt. Squeeze the juice of half a lemon, add a pinch of salt. Apply the resulting solution to the product. Lemon juice usually does the job of cleaning.
Read also: Where can you find sodium tetraborate
The chosen method should depend on the general condition of the brass product: the presence of oxidation or severe darkening indicates the need to use chemicals. If the contamination is insignificant, then first you should try cleaning with natural compounds.
CITRIC ACID AND SALT
In this case, lemon juice (ready concentrate) is mixed with table salt in the amount of 1-2 tablespoons and the resulting mixture is rubbed on the surface of the brass. When cleaning brass at home in this manner, care must be taken as the acid can deteriorate the condition of the surface if it comes into excessive contact. Table salt can be replaced with washing powder.
HOW TO CLEAN BRASS FROM OLD VARNISH
how to clean brass at home from oxide and other household contaminants were discussed above . However, an important question is: how to clean brass from old varnish at home and how to restore the protective coating.
Removing old varnish from brass products:
- Prepare your workplace. To do this, you need to cover the workspace with oilcloth;
- The old varnish coating is removed with nail polish remover, which is evenly applied to the surface using a soft brush. The procedure is carried out wearing protective equipment and taking into account its flammability;
- After removing the varnish, the surface is thoroughly polished using specialized pastes and polishes.
The prepared product is coated with a fresh layer of varnish. Brushes and cotton pads are used for this. It is necessary to ensure that the varnish is distributed evenly over the entire surface and does not form smudges or unpainted areas. In addition, you should avoid contact of your fingers with a wet surface to avoid fingerprints getting stuck. After applying the varnish, the product is dried and polished.
RESULT: HOW AND WHAT TO CLEAN BRASS AT HOME
Having considered all the options and methods for cleaning brass products, we can conclude that the most appropriate is to use professional products made taking into account all the nuances of such surfaces. If the alloy needs to be cleaned, and nothing other than “folk remedies” is at hand, the best results can be expected from aggressive, acidic environments. They must be used carefully, following all the rules and not too often. Products containing abrasives are used extremely rarely and carefully to avoid scratches.
RECOMMENDATIONS FOR CLEANING BRASS
What is contraindicated when cleaning brass:
- Use of coarse abrasive; Frequent use of citric acid, as it is the most aggressive of the acids towards brass surfaces; Clean with coarse cloths and brushes with a high degree of rigidity.
How to prevent excessive contamination of surfaces:
- Dry cleaning of products is carried out at least once every 7 days;
- Try to avoid direct sunlight on the surface of the products;
- Do not store brass items in areas with high humidity or in close contact with other metals.
In difficult cases, when it is not possible to carry out complete cleaning using available means without the risk of damaging the product, it is recommended to seek help from specialists.
Polishing a cleaned product
The final step in the cleaning process is polishing the product. Polish the item using a dry cloth made from natural materials and non-abrasive chemicals. Instead of chemicals, you can use a “light” solution of flour, salt and 100 ml of vinegar water. For the solution you will need 100 g of salt, 100 g of flour and 100 ml of vinegar solution. Rub the solution on the surface of the object in a circular motion, but carefully monitor its changes. Vinegar can ruin things, so be careful.
Group: Forum Participants Messages: 28 Registration: 9.2.2011 From: Moscow User No.: 93566
Shut-off ball valves made of brass (sometimes made of bronze or stainless steel) are usually installed on the outlets of hot water and hot water risers. As you know, steel pipes and brass taps have different electrochemical potentials, therefore, at the steel-brass transition point, electrochemical corrosion may occur, i.e. the steel of the pipe will collapse, because is the anode. Perhaps because of this, corrosion build-ups can often be seen on steel pipes in front of the taps, narrowing the passage.
As practice shows, the process of electrochemical corrosion can occur especially quickly on the outlets of risers of heated towel rails, perhaps due to the fact that water constantly flows through the heated towel rails and at one of the outlets the water flow is reversed (from the tap towards the riser), so one of the outlets is destroyed faster, as far as I understand, this is precisely the outlet where the water flow is reversed.
Also, as practice shows, the rate of destruction also depends on the quality (composition) of water, because At different objects the rate of destruction is different.
The question is: what documents regulate the use of taps made of brass (and other metals) on ordinary black and galvanized pipes? And what documents regulate water quality requirements?
Read also: Makita hammer drill instruction manual
Group: Forum Participants Messages: 1582 Registration: 12/26/2011 From: Novosibirsk User No.: 134454
Group: Moderators Messages: 7696 Registration: 1/17/2006 From: Chisinau User No.: 1877
Group: Forum Participants Messages: 856 Registration: 6/18/2007 From: Crimea User No.: 9559
Group: Forum Participants Messages: 43 Registration: 8.4.2010 User No.: 51301
Shut-off ball valves made of brass (sometimes made of bronze or stainless steel) are usually installed on the outlets of hot water and hot water risers. As you know, steel pipes and brass taps have different electrochemical potentials, therefore, at the steel-brass transition point, electrochemical corrosion may occur, i.e. the steel of the pipe will collapse, because is the anode. Perhaps because of this, corrosion build-ups can often be seen on steel pipes in front of the taps, narrowing the passage.
As practice shows, the process of electrochemical corrosion can occur especially quickly on the outlets of risers of heated towel rails, perhaps due to the fact that water constantly flows through the heated towel rails and at one of the outlets the water flow is reversed (from the tap towards the riser), so one of the outlets is destroyed faster, as far as I understand, this is precisely the outlet where the water flow is reversed.
Also, as practice shows, the rate of destruction also depends on the quality (composition) of water, because At different objects the rate of destruction is different.
The question is: what documents regulate the use of taps made of brass (and other metals) on ordinary black and galvanized pipes? And what documents regulate water quality requirements?