Carbon steels
Carbon steels at room temperature, as already mentioned, have low electrical resistivity due to their high iron content. At 20°C, the value of their resistivity is in the range from 13·10 -8 (for steel 08KP) to 20·10 -8 Ohm m (for U12).
When heated to temperatures above 1000°C, the ability of carbon steels to conduct electric current is greatly reduced. The resistance value increases by an order of magnitude and can reach a value of 130·10 -8 Ohm·m.
Electrical resistivity of carbon steels ρe·10 8 , Ohm m
| Temperature, °C | Steel 08KP | Steel 08 | Steel 20 | Steel 40 | Steel U8 | Steel U12 |
| 12 | 13,2 | 15,9 | 16 | 17 | 18,4 | |
| 20 | 13 | 14,2 | 16,9 | 17,1 | 18 | 19,6 |
| 50 | 14,7 | 15,9 | 18,7 | 18,9 | 19,8 | 21,6 |
| 100 | 17,8 | 19 | 21,9 | 22,1 | 23,2 | 25,2 |
| 150 | 21,3 | 22,4 | 25,4 | 25,7 | 26,8 | 29 |
| 200 | 25,2 | 26,3 | 29,2 | 29,6 | 30,8 | 33,3 |
| 250 | 29,5 | 30,5 | 33,4 | 33,9 | 35,1 | 37,9 |
| 300 | 34,1 | 35,2 | 38,1 | 38,7 | 39,8 | 43 |
| 350 | 39,3 | 40,2 | 43,2 | 43,8 | 45 | 48,3 |
| 400 | 44,8 | 45,8 | 48,7 | 49,3 | 50,5 | 54 |
| 450 | 50,9 | 51,8 | 54,6 | 55,3 | 56,5 | 60 |
| 500 | 57,5 | 58,4 | 60,1 | 61,9 | 62,8 | 66,5 |
| 550 | 64,8 | 65,7 | 68,2 | 68,9 | 69,9 | 73,4 |
| 600 | 72,5 | 73,4 | 75,8 | 76,6 | 77,2 | 80,2 |
| 650 | 80,7 | 81,6 | 83,7 | 84,4 | 85,2 | 87,8 |
| 700 | 89,8 | 90,5 | 92,5 | 93,2 | 93,5 | 96,4 |
| 750 | 100,3 | 101,1 | 105 | 107,9 | 110,5 | 113 |
| 800 | 107,3 | 108,1 | 109,4 | 111,1 | 112,9 | 115 |
| 850 | 110,4 | 111,1 | 111,8 | 113,1 | 114,8 | 117,6 |
| 900 | 112,4 | 113 | 113,6 | 114,9 | 116,4 | 119,6 |
| 950 | 114,2 | 114,8 | 115,2 | 116,6 | 117,8 | 121,2 |
| 1000 | 116 | 116,5 | 116,7 | 117,9 | 119,1 | 122,6 |
| 1050 | 117,5 | 117,9 | 118,1 | 119,3 | 120,4 | 123,8 |
| 1100 | 118,9 | 119,3 | 119,4 | 120,7 | 121,4 | 124,9 |
| 1150 | 120,3 | 120,7 | 120,7 | 122 | 122,3 | 126 |
| 1200 | 121,7 | 122 | 121,9 | 123 | 123,1 | 127,1 |
| 1250 | 123 | 123,3 | 122,9 | 124 | 123,8 | 128,2 |
| 1300 | 124,1 | 124,4 | 123,9 | — | 124,6 | 128,7 |
| 1350 | 125,2 | 125,3 | 125,1 | — | 125 | 129,5 |
High alloy steels
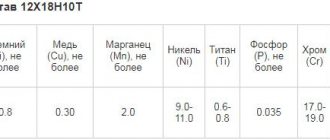
High-alloy steels have electrical resistivity several times higher than carbon and low-alloy steels. According to the table, it can be seen that at a temperature of 20°C its value is (30...86)·10 -8 Ohm·m.
At a temperature of 1300°C, the resistance of high- and low-alloy steels becomes almost the same and does not exceed 131·10 -8 Ohm·m.
Electrical resistivity of high-alloy steels ρe·10 8 , Ohm m
| steel grade | 20 | 100 | 300 | 500 | 700 | 900 | 1100 | 1300 |
| G13 | 68,3 | 75,6 | 93,1 | 95,2 | 114,7 | 123,8 | 127 | 130,8 |
| G20H12F | 72,3 | 79,2 | 91,2 | 101,5 | 109,2 | — | — | — |
| G21X15T | — | 82,4 | 95,6 | 104,5 | 112 | 119,2 | — | — |
| Х13Н13К10 | — | 90 | 100,8 | 109,6 | 115,4 | 119,6 | — | — |
| Х19Н10К47 | — | 90,5 | 98,6 | 105,2 | 110,8 | — | — | — |
| P18 | 41,9 | 47,2 | 62,7 | 81,5 | 103,7 | 117,3 | 123,6 | 128,1 |
| EH12 | 31 | 36 | 53 | 75 | 97 | 119 | — | — |
| 40Х10С2М (EI107) | 86 | 91 | 101 | 112 | 122 | — | — | — |
Low alloy steels
Low alloy steels are able to resist the passage of electricity slightly more than carbon steels. Their electrical resistivity is (20...43)·10 -8 Ohm·m at room temperature.
It should be noted that steel grades of this type are the worst conductors of electric current - these are 18Х2Н4ВА and 50С2Г. However, at high temperatures, the ability to conduct electric current among the steels listed in the table practically does not differ.
Electrical resistivity of low-alloy steels ρe·10 8 , Ohm m
| steel grade | 20 | 100 | 300 | 500 | 700 | 900 | 1100 | 1300 |
| 15HF | — | 28,1 | 42,1 | 60,6 | 83,3 | — | — | — |
| 30X | 21 | 25,9 | 41,7 | 63,6 | 93,4 | 114,5 | 120,5 | 125,1 |
| 12ХН2 | 33 | 36 | 52 | 67 | — | 112 | — | — |
| 12ХН3 | 29,6 | — | — | 67 | — | 116 | — | — |
| 20ХН3 | 24 | 29 | 46 | 66 | — | 123 | — | — |
| 30ХН3 | 26,8 | 31,7 | 46,9 | 68,1 | 98,1 | 114,8 | 120,1 | 124,6 |
| 20ХН4Ф | 36 | 41 | 56 | 72 | 102 | 118 | — | — |
| 18Х2Н4ВА | 41 | 44 | 58 | 73 | 97 | 115 | — | — |
| 30G2 | 20,8 | 25,9 | 42,1 | 64,5 | 94,6 | 114,3 | 120,2 | 125 |
| 12MH | 24,6 | 27,4 | 40,6 | 59,8 | — | — | — | — |
| 40Х3М | — | 33,1 | 48,2 | 69,5 | 96,2 | — | — | — |
| 20Х3ФВМ | — | 39,8 | 54,4 | 74,3 | 98,2 | — | — | — |
| 50S2G | 42,9 | 47 | 60,1 | 78,8 | 105,7 | 119,7 | 124,9 | 128,9 |
| 30N3 | 27,1 | 32 | 47 | 67,9 | 99,2 | 114,9 | 120,4 | 124,8 |
Chromium stainless steels
Chromium stainless steels have a high concentration of chromium atoms, which increases their resistivity - the electrical conductivity of such stainless steel is not high. At normal temperatures, its resistance is (50...60)·10 -8 Ohm·m.
Electrical resistivity of chromium stainless steels ρe·10 8 , Ohm m
| steel grade | 20 | 100 | 300 | 500 | 700 | 900 | 1100 | 1300 |
| X13 | 50,6 | 58,4 | 76,9 | 93,8 | 110,3 | 115 | 119 | 125,3 |
| 2Х13 | 58,8 | 65,3 | 80 | 95,2 | 110,2 | — | — | — |
| 3Х13 | 52,2 | 59,5 | 76,9 | 93,5 | 109,9 | 114,6 | 120,9 | 125 |
| 4Х13 | 59,1 | 64,6 | 78,8 | 94 | 108 | — | — | — |
Chromium-nickel austenitic steels
Chromium-nickel austenitic steels are also stainless, but due to the addition of nickel they have a resistivity almost one and a half times higher than that of chromium steels - it reaches a value of (70...90)·10 -8 Ohm·m.
Electrical resistivity of chromium-nickel stainless steels ρe·10 8 , Ohm m
| steel grade | 20 | 100 | 300 | 500 | 700 | 900 | 1100 |
| 12Х18Н9 | — | 74,3 | 89,1 | 100,1 | 109,4 | 114 | — |
| 12Х18Н9Т | 72,3 | 79,2 | 91,2 | 101,5 | 109,2 | — | — |
| 17Х18Н9 | 72 | 73,5 | 92,5 | 103 | 111,5 | 118,5 | — |
| Х18Н11Б | — | 84,6 | 97,6 | 107,8 | 115 | — | — |
| Х18Н9В | 71 | 77,6 | 91,6 | 102,6 | 111,1 | 117,1 | 122 |
| 4Х14НВ2М (ЭИ69) | 81,5 | 87,5 | 100 | 110 | 117,5 | — | — |
| 1Х14Н14В2М (ЭИ257) | — | 82,4 | 95,6 | 104,5 | 112 | 119,2 | — |
| 1x14N18M3T | — | 89 | 100 | 107,5 | 115 | — | — |
| 36Х18Н25С2 (ЭЯ3С) | — | 98,5 | 105,5 | 110 | 117,5 | — | — |
| Х13Н25М2В2 | — | 103 | 112,1 | 118,1 | 121 | — | — |
| Х7Н25 (ЭИ25) | — | — | 109 | 115 | 121 | 127 | — |
| Х2Н35 (ЭИ36) | 87,5 | 92,5 | 103 | 110 | 116 | 120,5 | — |
| H28 | 84,2 | 89,1 | 99,6 | 107,7 | 114,2 | 118,4 | 122,5 |
Heat-resistant and heat-resistant steels
In terms of their electrical conductive properties, heat-resistant and heat-resistant steels are close to chromium-nickel steels. The high content of chromium and nickel in these alloys does not allow them to conduct electric current, like ordinary carbon alloys with a high concentration of iron.
The significant electrical resistivity and high operating temperature of such steels make it possible to use them as working elements of electric heaters. In particular, steel 20Х23Н18 in its resistance and heat resistance in some cases can replace such a popular alloy for heaters as nichrome Х20Н80.
The resistivity of metals is their ability to resist electric current passing through them. The unit of measurement for this quantity is Ohm*m (Ohm-meter). The symbol used is the Greek letter ρ (rho). High resistivity values mean poor conductivity of electrical charge by a particular material.
Metal compatibility or how to avoid galvanic corrosion?
Contact corrosion occurs when two dissimilar metals come into direct contact.
It is impossible, for example, to connect aluminum sheets with a copper rivet, since under certain conditions they form a strong galvanic couple. Different metals have different electrode potentials. In the presence of an electrolyte, one of them acts as a cathode and the other as an anode. As a result of the chemical reaction occurring between them, a corrosion process will begin in which copper (cathode) will mercilessly destroy aluminum (anode).
Almost all pairs of dissimilar metals that are in contact with each other are subject to corrosion, since even moisture from the air can act as an electrolyte and activate their electrode potential. But some couples are more vulnerable, while others are less vulnerable.
For example, aluminum has excellent contact with galvanized steel, chromium and zinc, but brass is not at all “friendly” with steel, aluminum and zinc. To find out which metals are compatible and which are not, let's look at the basics of chemistry.
In the series of electrochemical activity, metals are in the following sequence:
For example, consider a pair of aluminum and copper. Aluminum is in the row to the left of hydrogen and has an electronegative potential of -1.7V, and copper is to the right and has a positive potential of +0.4V. A large potential difference leads to the destruction of more active aluminum. Copper is stronger than all the elements in front, so if paired with any of them, it will emerge victorious. The farther apart the elements are in a row, the higher their incompatibility and the likelihood of galvanic corrosion.
Data on the compatibility of some metals are presented in the table:
Aluminum
| Copper | Cink Steel | Iron | Lead | Stainless steel | Zinc | ||||
| Aluminum | D | N | N | N | D | ABOUT | ABOUT | D | D |
| Copper | N | ABOUT | ABOUT | D | ABOUT | N | ABOUT | N | N |
| Cink Steel | D | ABOUT | ABOUT | ABOUT | D | ABOUT | D | ABOUT | D |
| Lead | ABOUT | ABOUT | ABOUT | ABOUT | D | D | D | ABOUT | D |
| Stainless steel | D | N | N | N | ABOUT | ABOUT | ABOUT | D | N |
| Zinc | D | N | N | N | D | N | D | N | D |
D
– absolutely acceptable contacts (low risk of GC);
O
– limited permissible contacts (average risk of GC);
N
– unacceptable contacts (high risk of GC).
The table below can serve as a quick reference for determining the compatibility of certain structural metals. The permissibility and impermissibility of contacts between electrochemically dissimilar metals is established by GOST 9.005-72.
Steel Specifications
Before considering the resistivity of steel in detail, you should familiarize yourself with its basic physical and mechanical properties. Due to its qualities, this material is widely used in the manufacturing sector and other areas of people’s lives and activities.
Steel is an alloy of iron and carbon, contained in an amount not exceeding 1.7%. In addition to carbon, steel contains a certain amount of impurities - silicon, manganese, sulfur and phosphorus. In terms of its qualities, it is much better than cast iron; it can easily be hardened, forged, rolled and other types of processing. All types of steels are characterized by high strength and ductility.
According to its purpose, steel is divided into structural, instrumental, and also with special physical properties. Each of them contains a different amount of carbon, thanks to which the material acquires certain specific qualities, for example, heat resistance, heat resistance, resistance to rust and corrosion.
A special place is occupied by electrical steels, produced in sheet format and used in the production of electrical products. To obtain this material, silicon is doped, which can improve its magnetic and electrical properties.
In order for electrical steel to acquire the necessary characteristics, certain requirements and conditions must be met. The material must be easily magnetized and remagnetized, that is, have high magnetic permeability. Such steels have good magnetic induction, and their magnetization reversal occurs with minimal losses.
The dimensions and weight of magnetic cores and windings, as well as the efficiency of transformers and their operating temperature depend on compliance with these requirements. The fulfillment of the conditions is influenced by many factors, including the resistivity of steel.
Copper resistivity
The relatively low resistivity of copper is an important, but not the only positive factor.
The widespread use of this material is explained by its reasonable cost and resistance to adverse external influences.
It is easy to create high-quality products of the required shape from it, which, without additional protection, retain functionality during long-term use in difficult conditions.
Various types of cable products are made from copper
Copper is the main material for conductors
The qualified selection of a suitable material is accompanied by a comprehensive assessment of several factors. The copper conductor is not damaged by corrosion because a protective layer of oxides forms on the surface.
Structural integrity is maintained at tight turning radii after repeated bending. The noted parameters are useful for equipping rooms with high humidity and laying lines of complex configurations.
However, the main advantage is the low resistance of copper wires. In addition to improving current conductivity while reducing losses during energy transmission, it should be noted that the weight and size of cable products is reduced compared to alternative options.
Resistivity of pure metals at low temperatures
Calculation of voltage drop in a cable
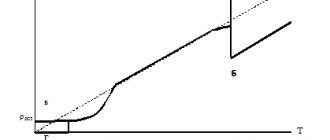
Vibrational processes in the molecular lattice prevent the free movement of electrons. This explains the increase in resistance as temperature increases.
A linear dependence is observed from a slight positive temperature, up to the melting point. The corresponding phase transition is accompanied by a sharp increase in electrical resistance.
Of course, such a regime after destruction is not working.
Sodium resistivity
Theoretical indicators “a” are confirmed by the results of experiment “b”. If the structure of a pure metal is distorted by impurities (impurities, alloy components), a random distribution of electric charge carriers will occur. This, in turn, will increase the circuit losses (resistance).
Metal resistance table
To be convinced of the benefits of copper, it is necessary to make an appropriate comparative analysis. Below are the metal resistance values in the summary table.
What is electrical resistance
Basic electrical parameters of conductors made from different materials
Material Resistivity in Ohms per meter, measured at room temperature (+20°C) Electrical conductivity under similar conditions, in Siemens per meter
| Copper | 1.68x10-3 | 5.96x107 |
| Silver | 1.59x10-3 | 6.3x107 |
| Gold | 2.44x10-3 | 4.1x107 |
| Aluminum | 2.82x10-3 | 3.5x107 |
| Tungsten | 5.6x10-3 | 1.79x107 |
| Iron | 1x10-7 | 1x107 |
| Platinum | 1.06x10-7 | 9.43x106 |
| Lithium | 9.28x10-8 | 1.08x107 |
Important! The low resistance of an iron conductor is not enough for the widespread use of corresponding products in practice. Active oxidation provokes rapid destruction.
Conductor resistivity table
In some situations, expenses are not considered. Military and space technology is created using conductors made of precious metals. Such solutions help reduce cross-section and weight, increase resistance to radiation and other special effects.
For the manufacture of serial products for household and industrial purposes, more affordable materials are used.
Data for calculating the electrical parameters of conductors taking into account temperature changes
Material Resistivity (in ohms per mm2/m) measured at room temperature (+0°C) Temperature correction factor (TC)
| Copper | 0,0176 | 0,004 |
| Aluminum | 0,0278 | 0,0045 |
| Steel | 0,13 | 0,0063 |
| Nikelin | 0,43-0,45 | 0,0072 |
| Brass | 0,04 | 0,002 |
| Nichrome | 0,98 | 0,0003 |
| Tungsten | 0,0612 | 0,00047 |
The use of stainless steel wire helps to increase strength while optimizing cost. To improve anti-corrosion properties, special additives are used. They increase the resistance of a steel conductor by almost 10 times compared to its copper counterpart.
In any case, the specific conditions during use, as well as the purpose of the products, are of particular importance. Nickel, for example, exhibits ferromagnetic properties at extremely low temperatures below the threshold “Curie point” (-358 0°C). Silicon, which is used to make microcircuits and transistors, has special semiconductor parameters.
Comparison of conductivity of copper and aluminum
The first conclusion can be made after studying the tabular data. Aluminum's resistance is approximately 80% higher than copper. Conductivity is worse in the same proportion. But for a correct analysis it is necessary to study additionally the following facts:
- aluminum is lighter, but to obtain similar electrical parameters you will need to increase the cross-section (conductor thickness);
- copper products (multi-core cables) are not damaged by repeated bending;
- The resistivity of aluminum changes more with increasing/decreasing temperature;
- a film of oxides forms faster on its surface, so for reliability (durability) modern wiring is made of copper.
Copper and aluminum cables are connected through a steel adapter to prevent electrochemical corrosion
Application of electrical conductivity of materials
The presence of the noted properties is used not only in engineering energy networks. Good electrical conductivity allows information signals to be transmitted over long distances without distortion.
Maintaining a high amplitude reduces the requirements for amplification paths and reduces the overall cost of systems.
Minimizing losses is useful in electrolysis installations, when creating contact groups and motor windings.
Important! In all of the above examples, in addition to a general increase in efficiency, you can count on preventing overheating.
Resistance calculation
To correct temperature changes, the last column of the second table shows separate multipliers for each position. The calculation is performed using the formula RT = Rn * (1+PC*T), where the given symbols mean:
- RT – electrical resistance in Ohms at a certain temperature;
- Rn – conductor resistance at zero temperature;
- PC – correction factor;
- T – operating temperature in degrees Celsius.
Concept of electrical resistance
This term refers to the property of creating obstacles to the passage of electric current in a circuit. The relationship between physical quantities is described by the classical formula R=U/I (symbols for resistance, voltage and current, respectively). The movement of electrons occurs under the influence of an electromagnetic field, a potential difference.
Any distortion of the crystalline structure of the molecular lattice increases the resistance of metals. This reason explains the strong dependence of the parameter on the purity of the material and temperature. Thus, standards for pipe products allow the use of various alloys.
Electrical copper (grade M006) is created with a controlled amount of foreign impurities of no more than 0.1%.
The qualified use of this material is preceded by an assessment of all significant factors. In addition to the cost, they clarify:
- features of mechanical and other types of processing;
- stability of electrical parameters under certain operating conditions;
- resistance to external influences, durability.
In some situations, the significant initial investment is justified by extended service life and reliability.