What is phosphoric acid
The substance belongs to the category of inorganic chemical compounds with the consistency of syrup. Its crystals quickly dissolve in ethyl alcohol and water.
The product is considered an antioxidant and is used as an acidity regulator. When used as an additive, it is designated with the code E338.
Description and chemical properties
Phosphoric acid has the formula H3PO4 with a molecular weight of 98 g/mol. Microparticles of the substance connect oxygen and hydrogen atoms. The concentration of components in the composition looks like this:
| Item name | Number of atoms | Mass percentage |
| Phosphorus | 1 | 65,3 |
| Hydrogen | 3 | 3,1 |
| Oxygen | 4 | 31,6 |
The orthophosphorus compound has a number of chemical properties. Among them:
- Rapid dissolution in water and ethanol.
- Formation of 3 rows of salts - phosphates.
- Causing burns when exposed to skin.
- Formation of explosive hydrogen when interacting with metal surfaces.
- Incompatible with hot surfaces, quicklime, alcohol-containing compounds, chlorates and pure metals.
- Melting at 42.35°C, but no decomposition.
Phosphoric acid is joined by oxygen and hydrogen atoms.
Properties
H3PO4 is a tribasic acid of medium strength. When interacting with a very strong acid, for example, perchloric HClO4, phosphoric acid exhibits signs of amphotericity - phosphoryl salts are formed, for example [P(OH)4]ClO4.
In aqueous solutions it undergoes electrolytic dissociation in three stages with the formation of hydronium cation. The degree of dissociation and type depend on the pH of the solution:
A distinctive reaction of orthophosphoric acid from other phosphoric acids is the reaction with silver nitrate, which forms a yellow precipitate:
H 3 PO 4 + 3 A g NO 3 → A g 3 PO 4 + 3 HNO 3 PO_+3AgNO_\rightarrow Ag_PO_+3HNO_>>>
A qualitative reaction to the H2PO4 − ion is the formation of a bright yellow precipitate of ammonium molybdenum phosphate:
H 3 PO 4 + 12 [ NH 4 ] 2 M o O 4 + 21 HNO 3 → [ NH 4 ] 3 PM o 12 O 40 ⋅ 6 H 2 O ↓ + 21 NH 4 NO 3 + 6 H 2 O PO_+12 [NH_]_MoO_+21HNO_\rightarrow [NH_]_PMo_O_\cdot 6H_O\downarrow +21NH_NO_+6H_O>>>
Acid production
Acid can be obtained in 3 ways:
- As a result of hydrolysis of phosphorus pentachloride.
- From phosphate.
- Using the reaction of phosphorus with water.
The latter method involves a violent reaction, so phosphorus oxide is pre-treated with an acid concentrate heated to 200 °C.
In laboratory conditions
The industrial technology for producing H3PO4 consists of the following steps:
- Oxidation of phosphorus during combustion to the anhydride state.
- Hydration and absorption of the finished compound.
- Acid accumulation
- Collecting vapors from the gas fraction.
The acid is obtained by hydrolysis of phosphorus pentachloride.
In addition, in laboratory conditions the component is generated by reacting toxic white phosphorus with dilute nitric acid.
This method has a number of limitations and requires compliance with special safety precautions.
At home
The extraction method of production, which can be implemented in everyday life, consists of using the sulfuric acid decomposition reaction of salts with a yield of up to 95%. The manufacturing technology consists of the following stages:
- A consistency based on natural phosphate (pulp) and sulfuric acid is fed from the reservoir into the extractor - a special steel vat with a stirrer.
- Four-hour treatment and heating to 90 °C leads to the appearance of phosphoric acid and crystallization of calcium sulfate.
- Natural phosphate is separated using a perforated belt with a special filter coating. Passing through the holes of this canvas, the finished product is sent to vacuum chambers.
Receipt
Phosphoric acid is obtained from phosphates, for example by treating calcium phosphate (the mineral apatite) with sulfuric acid:
C a 3 ( PO 4 ) 2 + 3 H 2 SO 4 → 3 C a SO 4 + 2 H 3 PO 4 (PO_)_+3H_SO_\rightarrow 3CaSO_+2H_PO_>>>
PC l 5 + 4 H 2 O → H 3 PO 4 + 5 HC l +4H_O\rightarrow H_PO_+5HCl>>>
Or by interacting with water phosphorus(V) oxide obtained by burning phosphorus in oxygen:
P 2 O 5 + 3 H 2 O → 2 H 3 PO 4 O_+3H_O\rightarrow 2H_PO_>>>
The reaction with water is very violent, so phosphorus(V) oxide is treated with a concentrated solution of orthophosphoric acid heated to 200 °C.
Molten orthophosphoric acid and its concentrated solutions have high viscosity, which is due to the formation of intermolecular hydrogen bonds.
Benefits of phosphoric acid
The use of a chemical compound for processing metal products is due to the effective dissolution of oxides and the formation of a durable protective film. The solution does not harm metal surfaces and converts traces of corrosion into iron phosphates.
Phosphoric acid converts traces of corrosion into iron phosphates.
Technical acid is actively used for washing heat exchangers, coils, heaters and products of the metal rolling industry, including the surfaces of pipes of water supply and heating systems, fittings, boilers, hydraulic structures and cast iron products. In addition, it is in demand for polishing surfaces.
The greatest demand is for zinc, which contains several additives - manganese, acid and zinc.
This mixture increases the protective properties of the film formed on the metal.
Safety precautions when working with acid
H3PO4 belongs to the class of hazardous compounds, so work requires strict adherence to a number of rules. Metal processing or other actions with acid are carried out in a special building with an organized supply and exhaust ventilation system, away from open flame sources.
To work with phosphoric acid, you need a respirator, gloves and goggles.
It is forbidden to start work without a respirator, gloves and goggles. You also need to provide a set of overalls and non-slip shoes.
If the substance comes into contact with the skin and eyes, or if vapors are inhaled, a person may experience a burn, a gag reflex, coughing and dizziness. To avoid complications, you must immediately remove contaminated clothing, rinse the affected area with running water, call a medical professional, apply a bandage and neutralize the spilled composition with an alkaline agent.
Storage rules
Orthophosphorus compounds are packaged with GOST 3885-73 markings. All requirements for storage and transportation are specified in the regulatory act.
The composition can be kept in steel tanks for the railway industry, tanks with a hermetically sealed lid, barrels, canisters, polyethylene and glass bottles, or in special rubber tankers. The packaged substance is allowed to be placed in covered hangars with heating. To preserve the physicochemical characteristics during melting, the crystallized form of H3PO4 is heated to 60°C. The shelf life, subject to all rules, is 3 years from the date of production.
Disposal of the compound is carried out in industrial conditions by neutralization with alkalis.
Shipping
There are a number of restrictions and requirements for transporting acid specified in GOST 3885-73. The composition can be transported on any transport using tightly closed containers.
Packaging requirements and rules for handling the substance
The container in which the acid is packaged must be marked “Dangerous” or “Corrosive Liquid”.
The additive can be stored and transported in the following packaging:
- polyethylene canisters;
- glass bottles;
- containers and tankers made of stainless steel, which has undergone special treatment;
- plastic cubes.
For convenience, the containers themselves are placed in polyethylene drums or wooden boxes, inside of which there should be soft filler to avoid damage to the packaging.
Contact of the substance on the skin or mucous membranes, in the eyes or in the respiratory tract can cause burns, nausea, vomiting, dizziness and tissue damage. In this case, you must immediately seek medical help.
Acid is dangerous to handle, so you can only work with it away from open sources of fire, in a well-ventilated area.
It is mandatory to have protective clothing: gloves, a respirator, goggles, boots and a suit for working with hazardous substances.
Use of phosphoric acid in medicine
In the dental field, the composition is used to treat the internal coating of crowns. This improves the adhesion of the product during prosthetics. The substance is used in pharmacology as an active ingredient in various medications or dental cement.
Phosphoric acid is used in the dental field.
An orthophosphorus compound can etch tooth enamel, which is required when filling.
Impact on human health
H3PO4 affects the acid-base balance, increasing stomach acidity. Therefore, foods high in the additive are contraindicated for people with gastritis and other problems caused by high pH levels. Some experts argue that acid leaches calcium from the body, worsening the condition of tooth enamel and bone tissue. With excessive consumption, gastrointestinal disorders, vomiting and nausea appear. In case of poisoning, you should immediately seek help from a doctor.
Structural formula
True, empirical, or gross formula: H3O4P
Chemical composition of phosphoric acid
| Symbol | Element | Atomic weight | Number of atoms | Mass percentage |
| H | Hydrogen | 1,008 | 3 | 3.1% |
| O | Oxygen | 15.999 | 4 | 65.3% |
| P | Phosphorus | 30.974 | 1 | 31.6% |
Molecular weight: 97.994
Orthophosphoric acid
(phosphoric acid) is a medium-strength inorganic acid with the chemical formula H3PO4, which under standard conditions appears as colorless hygroscopic crystals. At temperatures above +213 °C it turns into pyrophosphoric acid H4P2O7. Very soluble in water. Typically, orthophosphoric (or simply phosphoric) acid is called an 85% aqueous solution (a colorless, odorless, syrupy liquid). Also soluble in ethanol and other solvents.
Receipt
Phosphoric acid is obtained from phosphate: Ca3(PO4)2 + 3H2SO4 → 3CaSO4 + 2H3PO4 It can be obtained by hydrolysis of phosphorus pentachloride: PCl5 + 4H2O → H3PO4 + 5HCl Or by reacting phosphorus(V) oxide with water, obtained by burning phosphorus in oxygen: P2O5 + 3H2O → 2H3PO4 The reaction with water is very violent, so phosphorus(V) oxide is treated with a concentrated solution of orthophosphoric acid heated to 200 °C. Molten orthophosphoric acid and its concentrated solutions have high viscosity, which is due to the formation of intermolecular hydrogen bonds.
Properties
H3PO4 is a tribasic acid of medium strength. When interacting with a very strong acid, for example, perchloric HClO4, phosphoric acid exhibits signs of amphotericity - phosphoryl salts are formed, for example [P(OH)4]ClO4. In aqueous solutions it undergoes electrolytic dissociation in three stages with the formation of hydronium cation. The degree of dissociation and type depend on the pH of the solution: H3PO4(aq) + H2O(l) ↔ H3(aq)O+ + H2PO4(aq)- Ks1 = 6.9 * 10-3 H2PO4(aq)- + H2O(l) ↔ H3O+ + HPO4(aq)2- Ks2 = 6.2 * 10-8 HPO4(aq)2- + H2O(l) ↔ H3(aq)+ + PO4(aq)3- Ks3 = 4.7 * 10-13 A distinctive reaction of orthophosphoric acid from other phosphoric acids is the reaction with silver nitrate, which produces a yellow precipitate: H3PO4 + 3AgNO3 → Ag3PO4 + 3HNO3 A qualitative reaction to the H2PO4− ion is the formation of a bright yellow precipitate of ammonium molybdenum phosphate: H3PO4 + 12[NH4]2MoO4 + 21HNO3 → [NH4]3PMo12O40 * 6H2O + 21NH4NO3 + 6H2O
Phosphates
Salts of phosphoric acid are called phosphates. Phosphoric acid forms mono-, di- and tri-substituted salts. H3PO4 + NaOH → NaH2PO4 + H2O (sodium dihydrogen phosphate) H3PO4 + 2NaOH → Na2HPO4 + 2H2O (sodium hydrogen phosphate) H3PO4 + 3NaOH → Na3PO4 + 3H2O (sodium phosphate) Dihydrogen phosphates (monosubstituted phosphates) are acidic, hydrophosphates (dibasic phosphates) are weakly acidic local, medium (tribasic phosphates, or simply phosphates) - alkaline. Dihydrogen phosphates are usually highly soluble in water; almost all hydrogen phosphates and phosphates are slightly soluble. Calcination of salts leads to the following transformations: NaH2PO4 → NaPO3 + H2O 2Na2HPO4 → Na4P2O7 + H2O Phosphates do not decompose during calcination, with the exception of ammonium phosphate (NH4)3PO4. Organic phosphates play a very important role in biological processes. Sugar phosphates are involved in photosynthesis. Nucleic acids also contain a phosphoric acid residue.
Application
Used for soldering as a flux (on oxidized copper, on ferrous metal, on stainless steel), for research in the field of molecular biology. It is also used to clean rust from metal surfaces. Forms a protective film on the treated surface, preventing further corrosion. It is also used in the composition of freons, in industrial freezing units as a binder. Aviation industry
Contains hydraulic fluids NGZh-5U and its foreign analogues.
Food industry
Orthophosphoric acid is registered as a food additive E338.
Used as an acidity regulator in carbonated drinks, such as Coca-Cola. Agriculture
In fur farming (in particular, when raising minks), drinking a solution of orthophosphoric acid is used to prevent increased stomach pH and urolithiasis.
Dentistry
Orthophosphoric acid is used for etching (removing the smear layer) of enamel and dentin before filling teeth. When using 2nd and 3rd generation adhesive materials, tooth enamel must be etched with acid, followed by rinsing and drying. In addition to additional time spent on implementation, these stages carry the risk of various errors and complications. When applying orthophosphoric acid, it is difficult to control the degree and depth of demineralization of dentin and enamel. This leads to the fact that the applied adhesive does not completely (over its entire depth) fill the open dentinal tubules, and this, in turn, does not ensure the formation of a full-fledged hybrid layer. In addition, it is not always possible to completely remove phosphoric acid after it is applied to dentin. It depends on how the phosphoric acid is condensed. Residues of orthophosphoric acid impair the bonding strength and also lead to the formation of a so-called “acid mine”. With the advent of adhesive materials of the 4th and 5th generations, the technique of total etching (dentin - enamel) began to be used. In 6th and 7th generation adhesive systems there is no separate acid etching step, since the adhesives are self-etching. Although some manufacturers still recommend briefly etching the enamel to enhance adhesion even when using self-etching adhesives.
Application for metal
H3PO4 acid is widely used in metallurgy and in the processing of metal surfaces. With its help you can remove traces of corrosion and scale, transform rust or solder parts.
Rust removal
H3PO4 effectively eliminates corrosion from metal, including precious types (silver, brass, bronze), and also forms a thin protective layer that prevents the negative effects of external factors. After treatment with such a component, a cycle of corrosion and absorption of iron oxides begins on the surface, and then a gray oily film appears.
Phosphoric acid eliminates corrosion from metal.
Several technologies for removing oxides are used in industry and everyday life:
- Complete immersion of the part in acid.
- Surface washing using a brush, roller or spray.
- Treatment of the top surface, which is pre-cleaned mechanically.
To remove corrosion, the product is first degreased and washed. Next, an acid solution is prepared (100 g of 85% product is diluted in 1 liter of water), and the part is immersed in a tank with the composition for 1 hour. The contents should be stirred periodically.
After the treatment cycle is completed, the product must be removed and washed with a neutralizing agent. The following ingredients are used to prepare it:
- Water – 50%.
- Medical alcohol – 48%.
- Ammonia – 2%.
Rust converter
H3PO4 based rust converter forms a protective film on metal surfaces that prevents future corrosion processes. The connection is safe for the following sample types:
- Rolled metal products.
- Wells.
- Steam generators.
- Boilers and water heaters.
- Heating radiators.
- Heat exchangers.
- Serpentines.
- Heating and water supply units.
- Steam generators.
- Kotlov.
- Components of mechanisms and vehicles.
The rust converting agent is used for rolled metal products.
For soldering
Acid flux made from an orthophosphoric compound allows you to obtain high-quality soldering, provided that the remaining substance is immediately washed off with water. If you leave them to harden, a protective film will appear on the surface.
Use of phosphoric acid against rust
Phosphoric acid, whose effect on rust is widely known, can be used both on an industrial scale and to remove metal corrosion at home. Of course, such actions must be carried out taking into account the safety rules described above.
A clear advantage of phosphoric acid is that under the conditions of chemical cleaning from the surface of a metal using phosphoric acid, it is possible not only to remove loose oxidized masses, but also to create a small protective film on the surface of the metal product. The formation of such a film occurs as follows: the iron oxide is corroded and absorbed by the acid, instead the metal surface is phosphorized. People who carry out a similar cleaning procedure testify that after removing rust by using phosphoric acid, an oily film of a gray hue forms on the surface of the metal product.
At this stage, we can name several basic ways to combat the formation of oxides on metal surfaces:
- Metal etching, which involves complete immersion in an acid solution,
- Spraying the compound using a spray gun or applying it using a roller,
- Mechanical cleaning of metal from oxides followed by the use of acid.
The most suitable and effective method for cleaning metal from corrosion is selected in each specific case, taking into account the individual conditions in which the procedure can be carried out.
Phosphoric acid in everyday life
Acid is actively used in household chemicals to remove rust from bathroom surfaces. It is suitable for the care of earthenware and enamel coatings. Before distributing the solution, the surface is treated with a detergent composition, which is prepared from 200 g of the active ingredient and 1 liter of water.
Acid is used to remove rust from bathroom surfaces.
In this case, the compound cannot be used for processing acrylic parts.
Interaction of phosphoric acid
The properties of an inorganic substance determine its interaction with other substances and compounds. In this case, chemical reactions occur. The orthophosphorus composition interacts with:
- salts of weak acids;
- hydroxides, entering into a neutralization reaction;
- metals to the left of hydrogen in the activity series with the formation of salt and the release of hydrogen;
- basic oxides, participating in the exchange reaction;
- ammonium hydroxide, creating ammonium hydrogen phosphate;
- ammonia to produce acid salts.
Use of phosphoric acid in the food industry
In the food industry, H3PO4 is designated E338. The substance is used in the production of various products and to maintain their presentation for a long time.
What kind of additive is E338?
Food additive E338 is a pleasant-tasting inorganic composition that is a powerful antioxidant and maintains the initial color of edible products. The component is also used to acidify drinks and foods.
Dangerous or not
The food component is officially approved, but has a negative effect on the body in excess concentration. Studies have shown that an excess of the substance in drinks or foods can lead to:
- To the development of diseases of the gastrointestinal tract.
- The appearance of complications of gastritis or peptic ulcers.
- Violation of acid-base balance.
- Leaching calcium from the body.
- Development of caries.
- The appearance of vomiting.
Additive E338 may lead to the development of gastrointestinal diseases.
If you have any symptoms of intoxication, you should seek medical help.
Where and why is it added?
E338 is in demand in the production of powders for baking bread, melting cheeses, preparing sugar, cola, sprite, sausages, etc. The main area of application is the production of carbonated drinks. In addition, the supplement can be found in:
- Butter products.
- Processed cheeses.
- Sausages.
- Bulok.
- Milk.
- Cakes.
- Baby food.
The additive can be found in processed cheeses.
Food industry
The second most popular industry for the use of phosphoric acid is the food industry. Phosphoric acid is registered in the register of food additives of the Russian Federation under the name E338. The substance is positioned as safe (subject to the permissible amount for use).
The main properties characteristic of E338 are:
- acidity regulation
- preventing rancidity
- shelf life extension
- improvement of taste characteristics
- antioxidant effects
According to its properties, food grade orthophosphoric acid is a weak acid. It has the form of a colorless granular powder with excellent air absorption properties. In air it can “blur out”.
Most often, orthophosphoric acid food additive E338 is used in the following areas of the food industry: the production of sweet carbonated drinks, sausages, processed cheese, milk-containing products, processed cheese, and confectionery.
Taste: bitter with sourness.
E338 is used in such products as: Coca-Cola, Baikal drink, Pepsi-Cola, Coca-Cola light, 7 Days vanilla-cherry croissants.
Other applications
In addition to medicine, metallurgical and food industries, orthophosphorus chemical compound is in demand in organic synthesis. With its help, phosphorus salts of calcium, manganese, aluminum and sodium are created. Among other areas of activity where the component is found:
- Oil industry.
- Film production.
- Making matches.
- Production of fire-fighting and fire-resistant products.
Acid is no less important for agriculture, due to the beneficial effects of phosphorus on the condition of plants and the intensity of their growth.
Phosphoric acid is used in the manufacture of matches.
The component is necessary for farm animals, because improves metabolic processes and promotes the appearance of natural growths.
Preparation of phosphoric acid
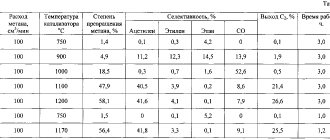
A chemical compound has several production methods. The well-known industrial method for producing phosphoric acid is thermal, which produces a pure, high-quality product. The following process occurs:
- oxidation during combustion with excess air of phosphorus to phosphorus anhydride having the formula P4O10;
- hydration, absorption of the resulting substance;
- condensation of phosphoric acid;
- capturing mist from the gas fraction.
There are two more methods for producing orthophosphorus compounds:
- An economical extraction method. Its basis is the decomposition of natural phosphate minerals with hydrochloric acid.
- Under laboratory conditions, the substance is obtained by reacting white phosphorus, which is toxic, with dilute nitric acid. The process requires strict adherence to safety precautions.
Application of phosphoric acid in agriculture
H3PO4 is an essential substance for agriculture. For this sphere, it is mined from special ore.
The importance of phosphorus for plants
Phosphorus is intended to maintain metabolism in plants and is an integral component of RNA and DNA. It is necessary for photosynthesis, regulation of respiration of green spaces and acceleration of seed germination. Without this chemical element, the normal formation of the root system, seeds and buds is impossible. Therefore, timely application of phosphorus to the soil is the key to the successful development of plants.
Phosphorus is necessary for photosynthesis.
If there is a deficiency of the element, the color of the leaves may change from dark green to crimson, they will begin to fall off and become covered in spots.
In addition, the plant will not be able to grow to its usual height, and root development will be suspended.
The use of phosphoric acid for fertilizing
For agricultural activities, acid is used, which is extracted from ore. Once in the soil, it helps plants cope with frost or drought. In addition, the chemical compound increases the fertility of the land, making it a favorable environment for the growth of various crops.
Vegetables
Phosphoric acid promotes the setting of fruits and flowers, therefore it is necessary for processing various vegetable crops. It is used when caring for cucumbers and tomatoes. The use of the substance in drip irrigation allows you to reduce the cost of purchasing fertilizer and cleaning irrigation systems.
Phosphoric acid is used in caring for cucumbers.
For trees and shrubs
When feeding shrubs and trees, H3PO4 can improve their appearance and stimulate fruiting. Fertilizing is carried out both in spring and autumn, using different proportions of components. The standard calculation for autumn work involves diluting 1 tsp. acids, 2 tsp. potash fertilizers in 10 liters of water.
For soil
Preparations based on H3PO4 have a beneficial effect on the condition of the soil, promoting its structuring, the proliferation of beneficial bacteria and the decomposition of important substances. In addition, the additive increases the acidity of the substrate, saturating it with mobile compounds of iron, manganese and aluminum.
Such components bind applied fertilizers, including non-granulated superphosphate.
Characteristics of orthophosphoric acid
Hearing the word acid, a person involuntarily tenses up, because even from old chemistry lessons in school years it is known that acid can have a fairly significant effect on objects or, for example, human skin. What is orthophosphoric acid? Is phosphoric acid, the use of which is recommended as one of the ways to combat rust, dangerous?
Orthophosphoric or simply phosphoric acid is presented in the form of a product of inorganic origin. At normal room temperature, orthophosphoric acid has the form of small diamond-shaped crystals.
Most often, orthophosphoric acid has the form of a syrupy 85% solution that has no characteristic odor. Orthophosphoric acid crystals dissolve quite well in water or ethanol.
Phosphoric acid equation
Phosphoric acid is used in the following sectors of human activity:
- Creation of fertilizers (phosphate),
- Production of special cleaning products belonging to the class of household chemicals,
- Dentistry,
- Substances to combat metal corrosion,
- Fur farming,
- Food industry.
If the ambient temperature, for example, in laboratory conditions exceeds 213 degrees Celsius, orthophosphoric acid is converted into pyrophosphoric acid. The composition of orthophosphoric acid and its chemical formula change accordingly.
Table 1. Physico-chemical parameters of orthophosphoric acid according to GOST 10678-76.
| Indicator name | Norm | ||
| Grade A | Brand B | ||
| 1st grade | 2nd grade | ||
| 1. Appearance | Colorless liquid transparent in a layer of 15-20 mm when viewed against a white background | Colorless or slightly yellowish liquid in a 15-20 mm layer when viewed against a white background | Colorless or colored liquid with a tint from slightly yellow to brown, not transparent in a layer of 15-20 mm when viewed on a white background |
| 2. Mass fraction of orthophosphoric acid (H3PO4), %, not less | 73 | 73 | 73 |
| 3. Mass fraction of chlorides, %, no more | 0,005 | 0,01 | 0,02 |
| 4. Mass fraction of sulfates, %, no more | 0,010 | 0,015 | 0,020 |
| 5. Mass fraction of nitrates, %, no more | 0,0003 | 0,0005 | 0,0010 |
| 6. Mass fraction of iron, %, no more | 0,005 | 0,010 | 0,015 |
| 7. Mass fraction of heavy metals of the hydrogen sulfide group, %, no more | 0,0005 | 0,002 | 0,005 |
| 8. Mass fraction of arsenic, %, no more | 0,0001 | 0,006 | 0,008 |
| 9. Mass fraction of reducing substances, %, no more | 0,1 | 0,2 | Not standardized |
| 10. Presence of metaphosphoric acid | Stands the test | ||
| 11. Mass fraction of suspended particles, %, no more | Stands the test | 0,3 | |
| 12. Presence of yellow phosphorus | Stands the test | Not standardized | |
Table 2. Physico-chemical parameters of orthophosphoric acid according to GOST 6552-80.
| Indicator name | Norm | ||
| Chemically pure (reagent grade) OKP 26 1213 0023 08 | Pure for analysis (analytical grade) OKP 26 1213 0022 09 | Clean (h.) OKP 26 1213 0021 10 | |
| 1. Appearance and color | Transparent, colorless liquid containing no suspended particles | ||
| 2. Mass fraction of orthophosphoric acid (H3PO4), %, not less | 87 | 85 | 85 |
| 3. Density P420, g/cm3, not less | 1,71 | 1,69 | 1,69 |
| 4. Mass fraction of residue after calcination, %, no more | 0,05 | 0,1 | 0,2 |
| 5. Mass fraction of volatile acids (CH3COOH), %, no more | 0,0004 | 0,0010 | 0,0015 |
| 6. Mass fraction of nitrates (NO3), %, no more | 0,0003 | 0,0005 | 0,0005 |
| 7. Mass fraction of sulfates (SO4), %, no more | 0.0005 | 0.002 | 0.003 |
| 8. Mass fraction of chlorides, (Cl)%, no more | 0.0001 | 0.0002 | 0.0003 |
| 9. Mass fraction of ammonium salts (NH4), %, no more | 0,0005 | 0,002 | 0,002 |
| 10. Mass fraction of iron (Fe), %, no more | 0,0005 | 0,001 | 0,002 |
| 11. Mass fraction of arsenic (As), %, no more | 0.00005 | 0.0001 | 0.0002 |
| 12. Mass fraction of heavy metals (Pb), %, no more | 0,0005 | 0,0005 | 0,001 |
| 13. Mass fraction of substances reducing KMnO4 (H3PO3), %, no more | 0.003 | 0.005 | 0.05 |
Types of phosphate fertilizers and their use
It is recommended to use all phosphorus preparations when digging the bed in the autumn, and not for surface distribution. This is due to the fact that the main active component (phosphorus) is in a form that is difficult to digest, and during the winter it completely decomposes, enriching all parts of the plants.
Superphosphate
For many vegetable growers and gardeners, superphosphate is the best feeding option. It contains monocalcium phosphate, phosphoric acid, magnesium and sulfur. Simple (15-20%) and double (50%) superphosphate are available for sale. Both varieties are used for open soil of all types and greenhouses, for all plants.
Superphosphate is the best feeding option.
The main areas of application are the processing of cucumbers, grapevines, strawberries, tomatoes and flowers.
When planting vegetables, 15-20 g of fertilizer is added to each hole, and when planting trees or shrubs - 35-70 g. At the growing season, fertilizers are distributed in liquid form: 100 g of simple superphosphate is diluted in 10 liters of hot water, and then applied into the soil around the perimeter of the trunk circle in a volume of 0.5 liters for each bush.
If double superphosphate is used, the dosage is reduced by 2 times due to the increased concentration of phosphorus.
Ammophos
Top dressing is obtained as a result of neutralization of acid with the participation of ammonia. This provokes the appearance of ammonium phosphate and nitrogen - an indispensable component for plant development.
Decorative plantings are fed at the rate of 15-25 g of fertilizer per 1 square meter. m. Fruit crops and berry bushes - 20-35 g per 1 sq. m. m.
Diammofos
The fertilizer can be found on sale under the names ammonium hydrogen phosphate and diammonium phosphate. It contains 50% phosphorus and 18-20% nitrogen. Top dressing is intended to reduce soil acidity and can be used in combination with manure or bird droppings. In most cases, the composition is distributed over the bed when planting vegetable crops.
Diammophos is a groundbait that is used on marginal lands.
So, if potatoes are planted, add 1 tsp into each hole. granulated diammophos. When planting cucumbers and tomatoes in seedlings, add 1 tsp to the soil. fertilizers
Potassium metaphosphate
The drug is sold in the form of a white powder. It contains 55-60% phosphorus oxide and 35-40% potassium oxide.
The fertilizer is quickly absorbed in acidic soil and has a beneficial effect on crops that are susceptible to chlorine.
Phosphorite flour
This mineral complex consists of 20% phosphorus and 30% calcium. In addition, it contains a complex of microelements. Phosphorite flour is used when digging up the soil before planting garden crops, based on the calculation of 1.5-2 kg of substance per 10 square meters. m. The substance is added to the pit for preparing compost.
Phosphorite flour is a mineral phosphorus fertilizer.
Bone flour
The product is made from processed cattle bones. It contains 15-35% phosphorus, calcium and biologically active additives, including zinc, cobalt, iodine, manganese, iron, etc. Bone meal is used to process flowers and vegetables.
The substance is insoluble in water and is characterized by slow absorption by plants. This process takes from 5 to 8 months. Bone meal is suitable for treating acidic soils before planting in a dosage of 2-3 tbsp. l. per hole for vegetables or 60-100 g per 1 sq. m when planting shrubs. For fruit trees, a dosage of 200 g per 1 square meter is used. m.
Nitroammofoska
Nitroammofoska is a complex phosphorus fertilizer that contains not only phosphorus, but also a number of other important fertilizers necessary for the growth and development of plants.
Nitroammofoska – phosphorus fertilizer.
The fertilizer contains nitrogen and potassium, which are quickly absorbed by vegetables, trees and shrubs.
Precipitate
This agrochemical consists of 30% phosphorus, and is therefore intended to reduce soil acidity. With its help, the fertile layer is enriched and already growing crops are fed.
Plants react positively to the treatment, provided that all rules of use have been followed.
Application
Phosphoric acid is used in many areas, from industry to dental treatment. The product is used by craftsmen as a flux when soldering, to clean the metal surface from rust. Liquid is used:
- for scientific research in molecular biology;
- as a catalyst for organic synthesis processes;
- for creating anti-corrosion coatings on metals;
- in the production of fire-resistant impregnations for wood.
The substance is used:
- in the oil industry;
- in the manufacture of matches;
- for film production;
- for the purpose of protection against corrosion;
- to clarify sucrose;
- in the manufacture of medicines;
- in refrigeration units as a binder in freon;
- during mechanical processing for polishing and cleaning metals;
- in the textile industry in the production of fabrics with fire retardant impregnation;
- as a component in the production of chemical reagents;
- in veterinary medicine for the treatment of urolithiasis in minks;
- as a component for metal primer.
In the food industry
The use of phosphoric acid in food production has become widespread. It is registered in the register of food additives under code E338. When consumed in acceptable quantities, the substance is considered safe. The following properties of the drug are useful:
- preventing rancidity;
- acidity regulation;
- shelf life extension;
- preservation of taste characteristics;
- enhancing the effect of antioxidants.
Phosphoric acid as an acidifier, leavening agent, and antioxidant is used in the bakery, meat, and dairy industries. Used in the production of confectionery and sugar. The substance gives products a sour, bitter taste. Additive E338 is included in:
- processed cheeses;
- muffins;
- carbonated drinks - Pepsi-Cola, Sprite;
- sausages;
- buns;
- milk;
- baby food;
- marmalade;
- cakes
Research has shown that overconsumption of products containing phosphorus compounds, especially carbonated drinks, can lead to health problems. It is possible:
- leaching of calcium from the body, which can trigger the formation of osteoporosis;
- violation of the acid-base balance - the additive can increase its acidity;
- the appearance of gastrointestinal diseases;
- exacerbation of gastritis;
- destruction of tooth enamel;
- development of caries;
- the appearance of vomiting.
In the non-food industry
The use of phosphoric acid can be observed in many areas of production. This is often due to the chemical properties of the product. The drug is used for the manufacture of:
- combined, phosphorus mineral fertilizers;
- activated carbon;
- phosphorus salts of sodium, ammonium, manganese;
- fire retardant paints;
- glass, ceramics;
- synthetic detergents;
- fire-resistant binding components;
- non-flammable phosphate foam;
- hydraulic fluids for the aviation industry.
- What to do if you have a cold neck
- Blood Rh factor
- Belyashi dough - step-by-step recipes. How to prepare delicious belyashi dough quickly and easily, video
In medicine
Dentists use orthophosphorus composition to treat the inner surface of the crown. This helps improve its adhesion to the tooth during prosthetics. The substance is used by pharmacists to prepare medicines and dental cement. In medicine, the use of orthophosphorus compounds is associated with the ability to etch tooth enamel. This is necessary when using adhesive materials of the second or third generation for filling. Important points - after etching the surface it is necessary to:
- Rinse;
- dry.
Phosphorus-potassium fertilizers: advantages and rules of use
Phosphorus group fertilizers are applied deep into the soil, closer to the root system.
Potash fertilizers are suitable for any type of substrate. They are also introduced deeply, because... take a long time to be absorbed by plants. After such treatment, crops quickly receive carbon dioxide and become resistant to sudden temperature changes, pests and diseases.
Potassium group
The potassium group includes the following compounds:
- Potassium chloride (KCl). Berry bushes with increased susceptibility to chlorine (blueberries and strawberries) are subject to early treatment with the composition. It is recommended to apply fertilizing during routine digging of the soil.
- Potassium salt. It is a mixture of KCl with sylvinite. Capable of neutralizing high acidity of the soil.
- Potassium sulfate. Intended for processing berry and fruit crops. It contains no harmful additives. Together with other fertilizers, the substance is distributed throughout the garden bed in spring and autumn.
Phosphorus-potassium fertilizers are applied to the soil.
Phosphorus group
The category of phosphate fertilizers includes the following types:
- Superphosphate. Sold in powder or granule form in pale gray or black. Before use, the product is diluted with water, because it takes a long time to dissolve in the soil.
- Double superphosphate. It has identical chemical and physical properties as the previous version, but is available in a 2-fold dosage. To fertilize carbonate and chernozem substrates, it is applied together with other organic matter. The optimal consumption of double superphosphate is 200 g per 10 kg of organic matter.
Nitrogen-phosphorus fertilizers and their application
Nitrogen-phosphorus fertilizers are widely used in agriculture. They are necessary to stimulate plant growth, increase resistance to adverse factors, including frost, and also to saturate root crops with polysaccharides.
Urea urea
The composition is obtained by the synthesis of gaseous ammonia and carbon dioxide. It contains a high concentration of nitrogen. When applying fertilizer, you need to take into account the intensity of soil heating. The higher it is, the better the substance will be absorbed by plants.
Urea urea is obtained from the synthesis of ammonia and carbon dioxide.
Ammonium nitrate
Ammonium nitrate is enriched with easily digestible nitrogen, which is suitable for treating frozen soil when growing winter crops. The dosage depends on the purpose of the feeding:
- When digging the soil, 50 g of the substance are consumed per 1 square meter. m. area.
- When planting in a hole, the dosage is reduced to 20-30 g.
Ammonium sulfate
The nitrogen-containing preparation is in demand among summer residents and large agricultural producers. It does not harm plants, but belongs to class 3 danger to humans. Therefore, when distributing fertilizer, you need to use personal protective equipment.
Ammonium sulfate is in demand among summer residents.
Optimal dosage per 1 sq. m. is 40 g of feeding.
Precautions when treating surfaces
In small quantities and in a diluted state, orthophosphoric acid does not exhibit toxicity. This substance is allowed to be added to food products. It is present, for example, in Coca-Cola.
However, when working with pure reagent or high concentration solution, safety precautions must be observed . Phosphoric acid is an aggressive substance that irritates the integument, eyes, respiratory and digestive organs.
In extreme cases, burns and poisoning are possible.
If the concentrated converter comes into contact with the skin or eyes, immediately rinse the contact areas with plenty of water or saline solution. Do not wipe the skin with napkins or a towel, as this will increase the penetration of the substance into the tissues. If the damage is severe and causes pain, then to make you feel better, take a painkiller .
If liquid gets inside, first aid for a person before being examined by a doctor is to support breathing and drink plenty of fluids. If the acid is spilled, it is neutralized with alkaline solutions. When working with chemicals, it is necessary to wear personal protective equipment (overall clothing, gloves, goggles, respirator). Work must be carried out under a hood or in the open air.
Complex fertilizers and rules for their use
Flowering plants and vegetable crops require not only potassium, phosphorus and nitrogen, but also a number of important microelements. To apply them, ready-made complex formulations are used.
Calcium nitrate
It is a complex preparation that can be used both in a solid state when digging up the soil, and when diluted with water for foliar treatment and application under the root.
Calcium nitrate is a complex drug.
External spraying is practiced when caring for cucumbers. The solution is prepared from 2 g of powder diluted in 1 liter of water.
Copper sulfate
The substance is effective in preventing the appearance of pests or diseases. But when it penetrates into the soil, it binds phosphorus compounds, causing a deficiency of the chemical element. To avoid such consequences, you must follow the instructions for use.
Calculation of the amount and rate of application of orthophosphoric acid
H3PO4 acid is used to treat various crops. If a concentrated solution is selected, the dosage should not exceed 1 tbsp. l. for 10 l. If diluted, you need to use the appropriate proportions based on the composition.
For feeding trees and shrubs
For autumn treatment of trees and shrubs, a mixture of the following ingredients is used:
- 1 tsp. orthophosphorus compound;
- 2 tbsp. l. potash fertilizers;
- 10 liters of liquid.
Phosphoric acid is used to feed trees.
In spring, the proportions for berry bushes, such as blueberries, are slightly changed, using 1 tsp. acids, 2 tbsp. l. urea and 10 liters of liquid. The aqueous solution is suitable for treating wet soil and then adding water to the holes.
For feeding vegetables
Vegetables are fertilized using the following method: dilute 1 tsp in 10 liters of water. H3PO4, 1 tbsp. l. urea, 1 tbsp. l. potash fertilizer, 1 tsp. copper sulfate and 1 tsp. boric acid. A manganese solution is added to the composition to saturate the plants with manganese. Fertilizing is used 3 times per season with a frequency of 1 time in 10 days.
For cleaning a drip irrigation system
To disinfect drip irrigation systems, a low concentration of orthophosphorus compound is used (up to 0.6%). When cleaning, all safety requirements must be taken into account. Acid is added strictly to water, and not vice versa.
Review of effective phosphorus-containing fertilizers
There are many phosphorus-containing complexes available in the fertilizer market. But the following compositions are especially effective:
- Potassium monophosphate. Concentrated fertilizer that easily dissolves in water and consists of ¼ phosphorus. Due to the lack of nitrogen in the composition, it is suitable for treating any plants.
- Nitroammophoska. Complex mineral fertilizer with high efficiency. It can be used for any crops. The most positive results were observed when processing gray soils and chernozems.
- Sodium phosphate. Feeding with a high concentration of phosphorus (about 26%), which saturates plant roots with nutritional components and has a beneficial effect on crop yields.
Phosphates salts of phosphoric acids
What are phosphates? These are salts of phosphoric acids in which phosphorus is in the highest oxidation state (+ 5).
The main structural units in phosphate anions are PO4 tetrahedra. Orthophosphates are salts of orthophosphoric acid (H3PO4). There are intermediate or trisubstituted orthophosphates, for example. Ca3(PO4)2, MgNH4PO4, and acidic: hydroorthophosphates, or disubstituted, for example. Na2HPO4, CaHPO4, and dihydrogen orthophosphates, or monosubstituted ones, for example. Ca(H2PO4)2, NH4H2PO4. Among natural phosphates, the most common minerals are phosphorite Ca3(PO4)2 and apatite Ca5(OH, Cl, F) (PO4)3. Of the medium orthophosphates and hydroorthophosphates, only alkali metal and ammonium phosphates are soluble in water. Medium orthophosphates of thorium, titanium, zirconium, hafnium, tin and bismuth are insoluble even in strong acids. Dihydrogen phosphates dissolve quite well in water.
Alkali metal orthophosphates in aqueous solutions are partially hydrolyzed and... used for preparing buffer solutions. Medium orthophosphates do not decompose when heated: Na3PO4 melts at a temperature of 1340° C, Ca3(PO4)2 - at a temperature of 1610° C, except in cases where the cation is thermally unstable.
Medium orthophosphates soluble in water or in compounds can be converted into acidic ones by the action of a solution on them. Acid orthophosphates are also obtained by incomplete neutralization of orthophosphate acid with the corresponding oxides, hydroxides or carbonates. Orthophosphates, which are poorly soluble in water, are usually obtained by exchange reactions.