Everyone has heard about brass: some people have ancient relics from their great-grandmothers at home, while others are fond of collecting beautiful antique items. The history of brass began before our era, which speaks of its usefulness and necessity for humanity. Then, in ancient times, brass was an alloy of copper with galmay (zinc carbonate). In Ancient Rome, this metal was called “golden copper” because of its similarity to gold; Coins were minted from brass: sesterces and duponies.
In addition to the nickname “golden copper”, brass was also called the “eternal” metal. This is explained by a number of unique features and technical properties that it possesses, as well as a wide scope of its use. But brass became what it is now in the 18th century thanks to James Emerson. It was he who, by combining copper with the metal zinc , officially obtained this alloy.
The “eternal” metal is similar to bronze and has similar technical properties and characteristics:
- resistance to long-term friction;
- melting fluidity;
- corrosion resistance.
Types and advantages of brass products
Products made from brass are famous for their durability and wear resistance when cared for with care and properly coated. Coating often involves applying a top protective layer directly to the metal itself. The choice of protective layer depends directly on the conditions of use of the product. If we talk about buildings or plumbing, the coating materials in this case are zinc, aluminum, chromium, nickel, etc. Also, the protective layer can have a decorative function when it comes to products for interiors or luxury items. To do this, manufacturers can plate brass products with silver or gold by sputtering.
Does brass rust? No, it doesn’t. An important advantage of brass (even a classic alloy without impurities and additives) among other metals is that it does not rust, but only darkens, loses its mirror shine, and oxidizes. Therefore, this metal was widely used and is still used to make faucets, basins, bathtubs, buttons, dishes, orders, medals, figurines, candlesticks, frames for large mirrors or paintings, bases for glass tables, various decorations, etc.
Brass heated towel rail: advantages and disadvantages
Recently, vintage-style faucets made in colors of copper, brass or bronze have become fashionable. A good addition to such products would be a brass heated towel rail. Its installation will allow you to maintain the unity of the bathroom interior design and organize high-quality heating. This article will tell you what to look for when choosing a brass coil.
A little about the material
Brass is an alloy of two parts copper and one part zinc, supplemented with various additives. The latter are usually used nickel, tin, lead, aluminum and manganese. The ratio of additional elements in the alloy and the amount of each of them can affect some properties of brass, as well as change its color.
Important! The chemical composition of brass used for the manufacture of plumbing fixtures must comply with DSTU GOST 15527. Data on compliance is usually indicated in quality certificates, which should be requested from the seller of heated towel rails when purchasing.
The main advantage of brass is its high resistance to corrosion. That is why this metal is widely used for the production of taps, mixers and other plumbing elements. Actually, all the advantages of brass coils are determined by the chemical properties of this material.
Advantages of brass coils
In addition to high resistance to corrosion, brass has excellent thermal conductivity. Compared to stainless steel, from which bathroom coils are most often made, this indicator for brass is 4 times higher. You can verify this by opening any reference book on materials science. Accordingly, a brass heated towel rail will be a more efficient heating device than its steel or stainless steel counterpart.
Due to the high thermal conductivity of metal, it is not necessary to select a heat exchanger with a large area. For good heating of the bathroom, you can purchase a brass heated towel rail, the dimensions of which will be significantly smaller than the steel product. Even a small device can handle heating a bathroom. This may be useful for those who are arranging a small bathroom.
We advise you to find out how to connect a bathtub to the sewer with your own hands.
Read: what features does Alligator bath furniture have?
Also among the advantages of brass plumbing products, it is worth noting their high resistance to leakage currents (stray currents). This phenomenon can occur for two reasons:
- in the apartment or in the neighbors on the riser, the electrical equipment is grounded to the pipes;
- The wiring or electrical grounding system in the apartment or one of the neighbors is faulty.
These factors turn plumbing pipes into conductors. Of course, it is unlikely that you will get a serious electric shock through them. But constant exposure to electricity, combined with chemical factors, often leads to the appearance of electrochemical corrosion on plumbing lines. If the pipes and coil are made of steel, they will quickly become unusable. A heated towel rail made of brass simply will not take such an impact, and, therefore, will work longer than its counterparts made of other metals.
Selecting brass heated towel rails
Today in plumbing stores you can find quite a lot of coils made of non-ferrous metals, including brass. The assortment includes models of both domestic and foreign production. The latter are still much more common. But alas, they are not suitable for use in apartment buildings.
Like any heating device, a heated towel rail should be selected based on the characteristics of the system in which it will be built. European models (this is indicated in their documents and confirmed by practice) are designed for lower pressure than is used in our heating and water supply systems.
In addition, their design does not imply frequent pressure drops in the system, which, alas, are not uncommon in domestic utility systems.
All this leads to the fact that brass coils of foreign brands work very briefly in modern apartment buildings and soon after installation they begin to require replacement.
Important! An imported heated towel rail can be used in a private home with an autonomous heating and water supply system. For city apartments, domestic appliances will be more suitable.
When choosing products from domestic manufacturers, you should also focus on the constant pressure operating in the heating system of an apartment or house. Information about it can be obtained from the management company or housing department. Russian-made coils can be of two types:
- monotube (made from one pipe);
- polypipe (made from several pieces of pipeline).
The former are suitable for systems with operating pressures from 5.7 to 7.09 Pa. Composite models can be used if the pressure in the system varies from 2.37 to 4.3 Pa. It should be borne in mind that the larger the diameter of the device body, the less pressure it can withstand. The details for a specific heated towel rail model should be checked with the seller before making a purchase.
We recommend reading how to clean a bathroom faucet until it shines.
Read: what are the benefits of Colombo bath accessories.
Find out where to start when renovating your bathtub.
As for temperature conditions, brass products are quite capable of withstanding up to 110⁰C. This is quite enough for long-term operation in modern heating systems.
Methods and means for cleaning brass
In order to give the product its original appearance, you need to know how and with what to clean brass at home. When choosing a purchased cleaning product, be sure to pay attention to the composition and acids contained in it. Each of the acids interacts with metals differently, so the probability of destroying the protective layer along with oxidation is quite high.
What is not recommended to use
To avoid damage and destruction of the protective coating, you need to know about the products that are not recommended for use when cleaning brass. Therefore, first we list the substances that are dangerous to brass:
- Vinegar or acetic acid. When interacting with this acid, products made from “eternal” metal undergo dezincification and acquire a bright red color.
- Sandpaper. Even with the smallest abrasive size, sandpaper can not only scratch the item, but also remove part of the protective coating.
You need to be very careful with store-bought chemicals. Pre-cleaning is, of course, necessary. But before you start the process , you must be familiar with the components of the product. Strong chemicals can not only corrode and destroy the top layer, but also change its structure. Watch the brass and do not leave it in a chemical solution for a long time if you decide to clean it using chemicals.
Compositions that will not harm the surface
Before any cleaning, you should verify the composition of your product. Brass may be a metal, but it does not react to a magnet. Therefore, if your product is magnetic, then you should conclude that the composition contains impurities. This means that the cleaning method should be selected carefully to protect the product from damage . If you are completely sure of the composition of the product, then cleaning can be done using the following means:
- Oxalic (ethanedioic) acid or cleaning agents or detergents that contain it. In its pure form, oxalic acid requires 200 g per 10 liters of water. For such a solution, plastic containers are best suited, since metal containers can be exposed to acid. There are two ways to prepare this solution: in cold or hot water. The “cold” method involves completely immersing the product in a solution and periodically monitoring the cleaning process, since this method can take several days. The “hot” cleaning method involves using hot water for the solution. The temperature of the water that flows from the tap will be quite sufficient. Using higher temperature water increases the risk of damage to the paint or the top protective layer, if any. The product should be completely immersed in the solution, otherwise the edges located above the level of the solution will begin to oxidize very quickly due to the combination of acid vapors with oxygen. It is also necessary to maintain the temperature by placing a plastic container with the solution in a hot bath. The time for the first procedure is 20-40 minutes; if necessary, the process can be repeated, reducing the time the product remains in the solution. The prepared solution for subsequent procedures can be stored in plastic containers: bottles or buckets with lids.
- Acetone. A cotton swab is moistened with acetone and wiped over the entire surface of the product. But this method is not suitable for varnished brass products.
- Formic acid. Cleaning is less effective, but possible. 30% acid is sufficient for cleaning. The effect is lower due to the rapid weathering of the component, but, on the other hand, it is gentle and ensures the safety of the product.
- Salt. Ancient cleaning method: 1 tbsp. l. for 1 glass of whey.
- Solutions of ammonia and ammonium carbonate. 10−15% is enough.
- Lemon juice with salt. Squeeze the juice of half a lemon, add a pinch of salt. Apply the resulting solution to the product. Lemon juice usually does the job of cleaning.
Brass: what it is, composition and properties of the alloy, areas of its application
Brass is a metal alloy based on copper (Cu) and zinc (Zn), to which nickel, lead, tin, aluminum, and manganese can be added. Depending on the composition, the alloy acquires different properties and colors.
Despite the discovery of zinc, which is the main component of brass, only in the 16th century, it was known to man before our era. For example, the Romans alloyed copper with galma (zinc ore) and made various jewelry and thin-walled utensils from the alloy.
The production of the alloy spread to Central Asia, from where the products reached Rus', where the strength and brilliance of the material were also appreciated. And only after the discovery of zinc in 1746, it became possible for the appearance of brass in the form familiar to modern people. This happened on July 13, 1781 , when James Emerson registered the corresponding patent, so they say that brass was discovered 2 times.
Brass composition
The classic formula for brass is the ratio of copper and zinc as 1:2. It is this ratio that was mentioned at the turn of the 19th and 20th centuries in the encyclopedic dictionary of Brockhaus and Efron. In modern conditions, the amount of zinc added to copper can be significantly less, but, as a rule, does not exceed 30%, with the exception of technical alloys, in which the presence of 50% zinc is allowed. The more zinc is added, the lower the cost of the final material, since zinc itself is cheaper than copper.
Based on the composition of the alloy, they are distinguished:
- Two-component, the formula of which is quite simple and is a combination of copper and zinc in various proportions. Such an alloy, in accordance with GOST, is marked with the letter “L”, followed by a number indicating the percentage of copper content. For example, “L80”, that is, the alloy consists of 80% copper and 20% zinc.
- Multicomponent, containing additional elements called alloying elements, for example, tin, lead, aluminum, etc. The marking of such alloys depends on the elements contained in their composition, and the amount of zinc is calculated by subtracting the share of other elements from 100%. For example, a brass alloy consisting of 63% copper, 3% lead and 34% zinc would appear as "LS63-3".
Depending on the zinc content in the brass alloy, there are:
- Red, the zinc content of which is in the range of 5–20%
- Yellow, containing more than 20% zinc
Properties of brass
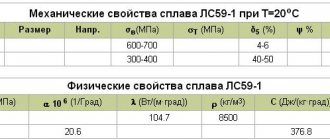
The melting point of brass is 880–950 °C , and the higher the zinc content, the lower the melting point. It is perfectly amenable to pressure treatment, has high mechanical properties, and good corrosion resistance.
However, for example, bronze outperforms brass in strength and corrosion resistance. It is also unstable in sea water, carbon dioxide solutions and organic acids. An unpleasant property of the alloy is its darkening in the open air; to prevent this, brass products are coated with varnish.
Brass parts do not lose their ductility when the temperature drops, which makes them a good structural material.
Brass and copper are very similar in appearance, and it will be difficult for a non-professional to differentiate between them. The first has increased hardness and wear resistance, but is less refractory . At the same time, brass alloy is much more convenient to process due to its high malleability and toughness.
It is also superior to copper in terms of corrosion resistance, and higher temperatures increase the rate of corrosion formation, the source of which can be high humidity, high levels of ammonia and sulfur dioxide in the air.
To prevent this, brass products must be fired at low temperatures after processing.
Properties of individual types of brass
Wrought brasses are alloys in which the zinc content is less than 10% ; they are also called tombak. Tompak is plastic, does not rust and has low friction force. Tompak welds well with steel and has a golden hue.
Foundry brass is intended for creating products by casting. copper in it varies from 50 to 80%. Such an alloy is not subject to rust, is not subject to deformation through friction with other materials, has good resistance to external force (high mechanical properties), and has no tendency to disintegrate. And also, due to its liquid state, the metal is easy to process, which allows you to pour it into any shape.
Automatic brass is an alloy in which lead is a mandatory element, which makes it possible to obtain short chips when processing a product in an automated mode, which reduces wear on the separating mechanism, increasing the speed of work.
The influence of alloying elements on the properties of the alloy
An alloying element is an element that is added to a metal to change its structure and chemical composition.
- Due to aluminum, a decrease in the volatility of the alloy is achieved, since a protective layer of aluminum oxide appears on the surface of the molten brass.
- Magnesium is usually used in combination with iron and aluminum to achieve increased strength and corrosion resistance of the product.
- Nickel protects the alloy from the negative effects of oxidation processes
- Lead is the most common alloying element, which increases ductility and malleability, as well as the quality of metal cutting.
- Silicon affects the strength and hardness of the alloy, and in combination with lead it increases anti-friction properties, which makes such an alloy competitive even with tin bronze.
- The addition of tin is due to the use of brass in sea water, as it increases the strength and anti-corrosion properties of the metal.
Application of brass
The alloy is one of the most used in the world, it is even called an eternal metal , since it is practically not subject to wear.
Two-component alloys containing up to 20% zinc are used to create coils, spare parts for machines, and thermal equipment. Compounds containing up to 40% zinc are used, for example, to create fittings and stamped products.
The use of multi-component brasses is much wider. They are used in the creation of pipes, ships, aircraft, clocks, springs, etc.
All kinds of insignia and artistic products are made from tombac. Various types of fittings, separators, bearings , and rust-resistant products are made from cast brass. The use of automatic brass is manifested in the creation of fasteners (nuts, bolts, screws, self-tapping screws, etc.), into which brass sheets, strips, and rods are cut.
Brass, which has the property of not being subject to magnetic attraction, is used to create compasses. Due to its high heat capacity, samovars were made from brass back in Tsarist Russia, which are still made from this material to this day.
Church items are also made from it.
Despite its low cost, the alloy is used to create prestigious things , for example, the popular Zippo lighters, the bodies of which are made of brass and then coated with other metals of various colors.
Brass in jewelry
The brass alloy has also found application in jewelry. Jewelers distinguish yellow (medium zinc content), golden (low zinc content), and green brass (high zinc content).
If the alloy consists of 15% zinc and 5% aluminum, then it closely resembles gold, and thanks to its excellent flexibility in polishing, a good craftsman will be able to make jewelry that a non-specialist will never be able to distinguish from a gold product.
This fact is also known to scammers who counterfeit gold. Oxalic acid is used to clean such jewelry.
Alloys marked “L62” and “L68” are the material on which novice jewelers are trained, since in terms of its mechanical characteristics it is as close as possible to gold.
Source: https://kamni.guru/ukrasheniya/metally/latun-chto-eto-takoe-ee-svoystva-i-primenenie.html
Polishing a cleaned product
The final step in the cleaning process is polishing the product. Polish the item using a dry cloth made from natural materials and non-abrasive chemicals. Instead of chemicals, you can use a “light” solution of flour, salt and 100 ml of vinegar water. For the solution you will need 100 g of salt, 100 g of flour and 100 ml of vinegar solution. Rub the solution on the surface of the object in a circular motion, but carefully monitor its changes. Vinegar can ruin things, so be careful.
Group: Forum Participants Messages: 28 Registration: 9.2.2011 From: Moscow User No.: 93566
Shut-off ball valves made of brass (sometimes made of bronze or stainless steel) are usually installed on the outlets of hot water and hot water risers. As you know, steel pipes and brass taps have different electrochemical potentials, therefore, at the steel-brass transition point, electrochemical corrosion may occur, i.e. the steel of the pipe will collapse, because is the anode. Perhaps because of this, corrosion build-ups can often be seen on steel pipes in front of the taps, narrowing the passage.
As practice shows, the process of electrochemical corrosion can occur especially quickly on the outlets of risers of heated towel rails, perhaps due to the fact that water constantly flows through the heated towel rails and at one of the outlets the water flow is reversed (from the tap towards the riser), so one of the outlets is destroyed faster, as far as I understand, this is precisely the outlet where the water flow is reversed.
Also, as practice shows, the rate of destruction also depends on the quality (composition) of water, because At different objects the rate of destruction is different.
The question is: what documents regulate the use of taps made of brass (and other metals) on ordinary black and galvanized pipes? And what documents regulate water quality requirements?
Group: Forum Participants Messages: 1582 Registration: 12/26/2011 From: Novosibirsk User No.: 134454
Group: Moderators Messages: 7696 Registration: 1/17/2006 From: Chisinau User No.: 1877
Group: Forum Participants Messages: 856 Registration: 6/18/2007 From: Crimea User No.: 9559
Group: Forum Participants Messages: 43 Registration: 8.4.2010 User No.: 51301
Shut-off ball valves made of brass (sometimes made of bronze or stainless steel) are usually installed on the outlets of hot water and hot water risers. As you know, steel pipes and brass taps have different electrochemical potentials, therefore, at the steel-brass transition point, electrochemical corrosion may occur, i.e. the steel of the pipe will collapse, because is the anode. Perhaps because of this, corrosion build-ups can often be seen on steel pipes in front of the taps, narrowing the passage.
As practice shows, the process of electrochemical corrosion can occur especially quickly on the outlets of risers of heated towel rails, perhaps due to the fact that water constantly flows through the heated towel rails and at one of the outlets the water flow is reversed (from the tap towards the riser), so one of the outlets is destroyed faster, as far as I understand, this is precisely the outlet where the water flow is reversed.
January 2016
| S | M | T | W | T | F | S |
| 1 | 2 | |||||
| 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 17 | 18 | 19 | 20 | 21 | 22 | 23 |
| 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| 31 |
Why copper and brass darken and how to care for jewelry
I have written more than once that, of course, brass and copper darken and oxidize
because they are not precious metals. I am often asked about this and I have never hidden the fact that jewelry can oxidize and darken over time. And you need to know this and be prepared for this when you purchase jewelry made from such non-traditional metals. After all, even silver oxidizes over time.
But! I want to point out that the reaction of metal to your body is very, very individual.
. I will speak in completely non-scientific simple words. It all depends mainly on the acidity level of your body. If the acidity is high, then copper and brass will definitely darken on you and leave dark marks, for example from rings and bracelets. If the acidity level is normal or reduced, then the jewelry you wear will not oxidize at all, much less leave marks, and will shine like new for a long, long time. I knew this before, but recently I proved it to myself experimentally.
I'm telling you :) I have always had a terrible sweet tooth and copper and brass have always oxidized on me. I have a copper swoosh ring (made by me, of course), which I always wear, never taking it off. I sleep, wash, live in it. And I used to wake up every morning with a black mark under this ring. And recently, almost a miracle happened - I almost lost interest in sweets))) Well, it’s not that I don’t eat sweets at all, but much less often and not even every day)). So my ring stopped oxidizing and leaving marks. I wake up in the morning and the ring is shining, my finger is clean and there is no dark streak on it. By the way, if anyone hasn’t understood yet, sugar acidifies the body, raises the level of acidity, and therefore copper and brass react to such changes. But as soon as I eat a chocolate bar or a cake, the next morning the ring darkens again, although not as much as before. It turns out to be a kind of litmus test) I, of course, do not encourage everyone to stop eating sweets at once so that the jewelry does not darken, but I think that it is useful to share such experience.
And now a few words about how to deal with this, how to care for jewelry made of copper and brass:
- For better preservation of jewelry, it is best to store it in a zip bag or closed box. They say that a piece of chalk placed in a jewelry box will protect it from oxidation; I haven’t tried it myself);
- After each wear, it would be good to wipe the jewelry with a rough woolen cloth. Or soft flannel;
- I recommend periodically thoroughly rubbing your jewelry with a silver cleaning cloth. It also works on copper and brass, but you will have to try a little longer to make the jewelry shine again;
- If the decoration has darkened, you can soak a piece of cloth in a solution of vinegar and table salt and wipe it thoroughly. Then rinse under running water;
- If the jewelry is not patinated and has fairly wide flat surfaces, then you can clean it with a toothbrush and a drop of toothpaste. But this must be done very carefully, especially if the decoration contains stones and small elements, so as not to damage them.