Brass is an alloy of copper and zinc. Other elements may be added. The more different components there are in brass, the cheaper it is. However, this alloy is stronger than pure metal. It does not oxidize so quickly and is not so plastic. And, most importantly, more affordable.
What is more expensive - copper or brass? The answer to this question is clear. Copper costs an order of magnitude higher than brass. The price may differ by two times. The reason is the composition on which the characteristics of these materials depend.
A LITTLE ABOUT COPPER
At its core, copper is a compound of crystals of silver, calcium, gold, lead and nickel. This combination is fundamental in acquiring certain properties.
Physical properties of copper:
- High softness index;
- High level of plasticity;
- Good ductility;
- High electrical and thermal conductivity.
Chemical properties of copper
- Low oxidation rates when used under standard conditions;
- Possibility of reacting with halogens, selenium and sulfur;
- Nitrogen, hydrogen and carbon do not react when they come into contact with copper;
- It dissolves in nitric acid concentrate, while dilute sulfuric and hydrochloric acids do not lead to a reaction.
Applications of copper
- Electrical engineering. Low resistivity allows the use of copper in the manufacture of power and other types of cables, wires and conductors of various directions. It must be taken into account that the presence of additional impurities can significantly reduce the electrical conductivity of the metal;
- Devices related to heat exchange. Copper's good thermal conductivity makes it suitable for cooling radiators, air conditioning and heating systems, computer systems and heat pipes;
- Pipe production. High levels of mechanical strength and ease of processing make copper a popular material in the production of seamless round pipes;
- Making dishes. Copper is most often used as a material for making decorative dishes and food storage containers;
- Decorations. The beautiful shade of copper and environmental friendliness make it an attractive material for the production of costume jewelry;
- Construction. Due to its high anti-corrosion properties, copper is successfully used as the main roofing material for residential buildings;
- As a ligature binder when working with other metals;
- Medicine. In medicine, copper is used as a component of some ointments and drops;
- Pyrotechnic devices.
Benefits of Copper
- High anti-corrosion properties;
- Aesthetically attractive appearance;
- High thermal conductivity;
- Good indicators of flexibility and ductility while maintaining strength;
- Low level resistance.
Flaws
High price. The main (conditional) disadvantage of copper can be considered the rather high cost of the material.
About copper, brass, tin, bronze and other alloys.
Due to my passion for jewelry, I became actively interested in what we make everything from - what kind of metals are they? what properties do they have? What is the gold standard in karats? What kind of turquoise do I buy? What types of fakes are there? etc. I thought that this is interesting not only to me... so I will slowly post it all here...
(all information is “pulled” from the Internet from different sites)
—————————————————————————————————————
Copper is a metal designated in the periodic table of chemical elements as Cu (Cuprum). Copper is one of the first metals that people began to use in ancient times. As a result, today all copper deposits have been selected, and it is mined from low-grade ores.
Man discovered copper before all other metals except gold. Even in prehistoric times, copper was used by Stone Age people.
Copper is found in a fairly pure state - in nuggets and grains of metal without impurities. Perhaps the first time a person picked up these nuggets from the ground was because they were beautiful. Then the man made a great discovery, finding out that these strange reddish stones can be given any shape. This was a simpler method of making weapons and knives than chipping flints.
Much time passed, and other people discovered that they could melt red stones and make cups and jugs from the molten mass. Then people began to mine copper and make all kinds of devices and utensils from it.
For thousands of years, copper remained the only workable metal because gold was not only too rare to be considered, but also too soft for practical purposes. Copper tools may have been used in the construction of the great pyramids of Egypt.
When bronze (an alloy of copper and tin) was discovered, even more copper was mined. But after the discovery of iron, copper began to be used in small quantities, mainly by peoples at a low level of civilization, until the era of electricity arrived. Since copper is a good conductor of electricity, it is widely used in modern industry.
Very few people have seen pure copper and are unlikely to recognize it if they see it. It is a shiny silvery substance with a slight pinkish tint, which turns reddish in color as it comes into contact with air. The copper we typically see is reddish-brown in color. This is the color of copper oxide, which is formed as a result of the interaction of metal with air.
Most of the world's copper exists in combination with other substances from which it must be separated before use. It is often adjacent to sulfurous substances, which can also be combined with iron and arsenic, which makes it difficult to purify copper.
Copper has some other advantages, besides the fact that it has outlived many other metals. It has high strength, but nevertheless is flexible enough that it can be stretched and given any shape through processing. It conducts heat no worse than electricity. Copper can be carved and engraved. But it's not easy to break. It can also be used to create alloys such as bronze and brass by combining it with other metals.
———————————————————————————————————————-
Brass (yellow copper) is one of the most useful and most commonly used alloys. Its composition varies over a fairly wide range depending on its purpose, but the main components - copper and zinc - are usually found in a ratio of about 2 parts copper and 1 part zinc. (Although zinc was discovered in the 16th century, brass was already known to the ancient Romans and was prepared by them by reducing the smelting of copper (or oxygen copper ores) with galmay, which was believed to have the property of turning copper yellow.This method of preparing brass was also practiced in the Middle Ages and survived until our century, but is now completely left). Brass sometimes contains trace amounts of tin and lead. Brass is harder than copper and therefore more difficult to wear; it is very malleable and viscous and therefore easily rolled into thin sheets, flattened under the blow of a hammer, drawn into wire or stamped into a wide variety of shapes; it melts and casts relatively easily at temperatures below the melting point of copper. Although the surface of brass, if not varnished, turns black when exposed to air, yet as a mass it is more resistant to the action of the atmosphere than copper. Finally, it has a beautiful yellow color and polishes well.
Many people do not wear brass jewelry because it can cause skin irritation and allergies. This occurs when nickel is added to brass. Yes, brass with the addition of nickel has a beautiful tint, it looks richer and more expensive, but it is these expensive brass jewelry that cause the most severe skin irritation. Tip: Buy cheap brass or look for the word Nickel free on the label.
——————————————————————————-
Tin is a relatively soft metal, used primarily as a safe, non-toxic, corrosion-resistant coating in its pure form or in alloys with other metals.
It is one of the seven metals of antiquity. In Egypt, Mesopotamia and other countries of the ancient world, bronze was made from tin already in the 3rd millennium BC. e.; tin was also used for making various household items, especially dishes.
Half of the tin mined worldwide is used to produce tinplate, used mainly for making tin cans. Therefore, tin is sometimes figuratively called the metal of a tin can.
The alloy of tin and copper itself - bronze , being a kind of symbol denoting a long period in the history of mankind - the Bronze Age, testifies to man's long-standing acquaintance with tin.
It is not so difficult to understand the reason why tin and copper became the object of attention of ancient people and why bronze played such a large role in the history of human culture. It is relatively easy to obtain copper from ores, but it is even easier to obtain metallic tin, whose melting point is only 232°C. It is enough to mix tin ore (the most important of them is cassiterite, or tin stone, a compound of tin with oxygen), set fire to the coal and blow the air with ordinary blacksmith bellows, which people used many thousands of years ago, so that pure tin is smelted. In any case, in Central Europe, where information about metals penetrated from the most ancient centers of culture, tin was known two thousand years BC. The Egyptians could obtain tin from ores as early as 3000 BC. The very name of tin (from the Sanskrit word “sta”, which means “hard”) indicates that in the countries of the East this metal was known even earlier, more than 4000 years BC.
It can be assumed that at first bronze was obtained by accident, since there are ores containing both tin and copper. Later, bronze was prepared according to a specific recipe, as evidenced by the results of the analysis of ancient bronze items.
Very often bronze contains lead and nickel. Be careful when choosing bronze jewelry and do not purchase it from dubious sources. Such jewelry can cause serious damage to health.
More than three hundred years ago it was noticed that tin adheres very well to the surface of pure iron and protects it from rusting. At the same time, from the experience of centuries-old use of tin utensils, it was known that tin almost does not tarnish and food in tin utensils does not acquire an unpleasant aftertaste.
Unlike early and medieval lead-containing alloys, modern cookware made from tin alloys is safe to use.
Pewter is a tin-based alloy. The old Russian name for pewter is table tin. Technical pewter contains lead and is harmful to humans. Jewelry pewters do not contain lead and nickel (Lead free and Nickel free). These alloys are ideal for casting, are easy to process, and gilding and silvering are ideal for pewter products. The Queen of England herself eats from dishes made of pewter. Also, amulets and talismans have been made from pewter since time immemorial, because this material, as it turned out, is very sensitive to human energy. Therefore, if you have pewter jewelry, do not let anyone wear it. This can lead to sad consequences for both you and the person to whom you lent your jewelry. The only serious drawback of the pewter is that it is quite fragile and breaks easily. Do not drop or bend these jewelry to avoid breakage.
——————————————————————————
Cupronickel (maillechort French). A very witty name given by the inventors Maillot and Chorier, as if an alloy of two names, which turned out to be consonant with the name of the biblical sorcerer Melchior (remember the worship of the Magi to the baby Jesus). When we say cupronickel, we mean an alloy of silver and copper with a low silver content. Is it so? In fact, this alloy consists of copper, nickel, manganese and iron. Where's the silver? He's not there. It looks very similar to silver: the same noble shade, resistance to corrosion. Medical instruments are even made from it. But it still does not contain silver.
————————————————————————-
Nickel silver (from German Neusilber - new silver), an alloy of copper with nickel and zinc. With a high nickel content, it has a beautiful white color with a greenish or bluish tint and high resistance to corrosion. Expensive products made from N-type alloys called “pakfong” were brought to Europe from China in the 18th century. In the 19th century products made from alloys of this type, usually silver-plated, were produced under different names: Chinese silver, cupronickel, etc. And there is also no smell of silver here.
A LITTLE ABOUT BRASS
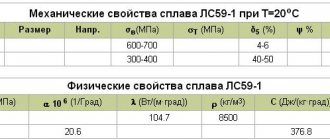
Brass is an alloy of copper and zinc. The percentage of zinc can vary from 5 to 45%. In some cases, the percentage of zinc content may slightly exceed 45%. The zinc in brass is intended to improve the quality of the metal, while significantly reducing its cost compared to the original material - copper.
Brass is divided into two main groups in its composition:
- Two-component. They consist of two components - copper and zinc. Moreover, the latter is the main binding component and usually makes up from 30 to 50%. Two-component brass containing up to 97 percent copper is called red. Their second name is “tompak”. Brass with a copper percentage not exceeding 35 is called yellow;
- Multicomponent. Alloys containing several alloy additives. The most commonly used amplifiers are manganese, tin, nickel, lead and silicon.
The marking of a brass alloy directly depends on the type and percentage of components. Thus, two-component brass is marked with alphabetic and numerical designations, where L denotes the material, and subsequent numbers indicate the percentage of copper content. Multi-component alloys have more complex markings, but the essence remains the same as simple brass.
Basic properties of brass
- Ease of processing under pressure;
- Good anti-corrosion resistance;
- High temperatures, aggressive environments, and exposure to sulfur dioxide increase the risk of corrosion;
- When temperatures drop, ductility increases, but strength does not decrease;
- When exposed to temperatures from 200 to 600 degrees, fragility increases significantly;
- Good anti-friction qualities;
- Good ability to weld with other metals.
Application areas of brass alloy
- Manufacturing of bushings and other transition parts;
- Production of components for motor units;
- Plumbing equipment and accessories;
- Decoration elements of various directions;
- Shipbuilding;
- Army needs.
Advantages of brass
When comparing brass with copper, there are common properties and characteristics:
- Ease of processing and polishing;
- Aesthetic appearance;
- Loyalty of tombak when welding with other metals;
- High anti-friction properties.
And yet: how to distinguish copper from brass ? There are many indicators by which you can see how copper differs from brass, such as the difference in temperature, color, hardness, etc...
Visual identification of differences
Considering brass and copper, the difference in external parameters is visible only in three ways:
- Color. Noticeable when studying alloys with different components. Brass compounds always have a golden or yellowish tint. Copper may be brownish, reddish or pinkish. The shade of brass alloys is influenced by the percentage of zinc content, and of copper alloys by its own proportion.
- Weight. The content of zinc and non-metallic substances in the composition of brass reduces the weight of products. Copper is heavier because it is made with a higher proportion of metal components. However, the indicator directly depends on the brand, so it does not provide accurate information.
- Shavings. When cutting a layer from copper, the waste will curl, since the metal is soft. There will be splinters flying off the brass.
These features do not allow us to give an accurate assessment of similar alloys, but they make it possible to easily identify the desired metal of different grades.
Inspection is usually required before selling metals on the secondary market. We will identify and evaluate products free of charge, guaranteeing fair prices. To find out the conditions, go to the “Acceptance of brass for scrap” page.
DIFFERENCE IN COPPER AND BRASS IN MELTING TEMPERATURE
The main difference between copper and brass in terms of temperature is that copper melts at a temperature of 1082 degrees, while most brass alloys melt at 880-1000 C. It is worth considering that the melting point of both materials is an indicator the differences can be considered conditional, due to the fact that the melting temperature of brass changes (increases and decreases) depending on the additives included in the alloy. It is almost impossible to achieve such conditions at home. In this regard, to carry out identification using high temperature, the heating method with a gas burner can be used. When the 600C mark is reached, a pale ashy coating of zinc oxide will appear on the surface of the brass alloy.
Learning to identify copper and distinguish it from other metals and alloys
Chemically pure copper has three distinctive characteristics. It is a colourful, ductile and corrosion resistant metal. The latter property is due to the formation of a thin oxide film. This layer makes the copper chemically inert in a non-corrosive environment and also adds a red tint to its golden pink color.
The best way to accurately identify copper is spectral analysis, which requires expensive equipment - a metal analyzer, while identifying copper at home is a task with a limited set of tools. Here the best instruments are the senses, easily accessible chemicals, fire and improvised devices.
How to distinguish copper from bronze
It is not always possible to determine which metal is in front of you by color. Bronze is an alloy of copper and tin, also characterized by a pink-red hue; scrap bronze can be found in anything. In this case, the main distinguishing characteristic is the ductility of the pure metal. By pressing on the copper with a hard object, we get a notch on the surface. Deforming bronze is much more difficult.
Products made of bronze are very difficult to distinguish visually from copper
An alternative way to distinguish copper from bronze at home is a saline solution. Add 200 grams of table salt to a metal container containing 1 liter of water. The solution is heated to a temperature above 50 °C. Next, the metal is placed in the heated liquid and kept for about 15 minutes. The color of copper changes. Bronze remains insensitive to the effects of saline solution.
The next method is patination of copper. Oxidation of pure metal over time in air is an inevitable process, leading to the formation of a greenish-blue coating. Bronze is not subject to patination.
How to distinguish copper from aluminum
Naturally, metals are easy to distinguish by color. The situation becomes more complicated when it is necessary to determine what the cable cores are made of. Tinned copper takes on a silvery tint, while copper-plated aluminum takes on a yellow tint. The result is that it is extremely difficult to distinguish metals from each other by color.
Tinned copper in cables
The best option is to measure the resistance. For a copper twisted pair cable about 100 meters long, the parameter value reaches 4–8 ohms. The resistance of a similar aluminum cable is significantly higher: 12 – 20 Ohms. This method is good because there is no mechanical impact on the metal.
The second method is bending/extension of the vein. The aluminum conductor will break quickly. The next option is a flame test. The melting point of aluminum is 600 °C, that of copper is much higher.
Other cases of fire and acid testing
Exposure to flame is used not only to identify metal relative to aluminum. For these purposes, a gas stove, lighter or fire is sufficient. Heating copper leads to the formation of its oxide, which affects the color change. The surface of the metal gradually becomes dull until it acquires a completely dark shade.
Nitric acid is another identifier for copper at home. It is also important to be careful here. It's better to just drip the liquid onto the metal. Pure copper at the point of contact will turn blue-green.
- how to distinguish aluminum from copper:
Finally
Before you begin to determine the material for making a part, you can carefully examine its surface. Many products are marked. It will help determine not only the type of metal, but also the brand.
DIFFERENCE OF COPPER AND BRASS IN SPECIFIC GRAVITY AND DENSITY
The brass alloy has a lower density compared to copper, and therefore the specific gravity will be less than that of copper. Determining which is heavier: copper or brass, if we are talking about a small volume, without using scales will be quite problematic.
Determining the type of material using a scale
- Determine density. This characteristic has fixed values and corresponds to 8.96 g/cm3 for copper and 8.73 g/cm3 for brass;
- Selection of necessary equipment. Such equipment will be: spring scales, tape measure, calculator and a cylindrical container with water;
- Determination of mass by weighing;
- Mass is converted to gram unit of measurement;
- Calculation of volume in cubic centimeters. For samples of the correct shape, it is necessary to measure the length, width and height, and multiply the values. For objects of irregular shape, the calculation is carried out by immersion in liquid and calculating the displaced volume;
- Calculation of density based on the data obtained. For this action, mass and volume are converted into the required dimensions and divided among themselves.
The results obtained are compared with standard indicators and become determining what kind of material the sample is.
How to determine what we have in front of us: brass or copper, their main differences
Anyone who searches for and sells non-ferrous metal sometimes has doubts about the type of scrap and, accordingly, its true value upon delivery.
Copper is a non-ferrous metal, and brass is an alloy that is typically 70% copper, so it often resembles it.
A mistake can be quite costly. For copper at collection points they give 285-300 rubles, for brass - about 150 . There are many ways to find out what kind of metal we see - copper or brass, and we will tell you how to distinguish them from each other in this article.
By product type
Some products are made only from copper or only from brass.
This may provide additional guidance.
Tools are made exclusively from brass, which is harder.
Some parts of wind musical instruments are made from copper.
In principle, you need to start from the purpose of the item - if it should be:
- reliable,
- hard,
- stiff,
then, most likely, brass .
If, on the contrary, you need ductility , high electrical or thermal conductivity, then this is copper .
Another method in which you need to use a gas burner.
The indicator here will be zinc oxide, which forms as a pale white ash-colored coating only on brass if it is heated to temperatures above 600 degrees .
HOW TO DISTINGUISH COPPER FROM BRASS BY COLOR
- If patina forms on the metal, it must be cleaned using non-aggressive household chemicals or by polishing;
- The object is placed in a source of bright white light, which can be a fluorescent lamp;
- As an analytical sample to facilitate the determination of copper and brass by color, you can use a polished copper 50-kopeck coin from 1997-2006. Copper with no impurities has a reddish-brown tint;
- Brass has a more yellow tint compared to copper. It is worth considering that there are brass samples with a high content of zinc impurities. This alloy will have a light gold or gray tint.
It is worth considering that this method of distinguishing copper from brass may not always be authentic.
So, for example, an alloy containing up to 85% copper component can be distinguished from pure metal only by a barely noticeable yellowish tint, while the main color will be similar to the color scheme of copper.
The main differences between copper scrap metal
If there is a certain amount of copper-like scrap accumulated around the house, the question arises: is it possible to make sure that it is really Cu? Is it worth taking scrap metal to a collection point? Is it possible to sell copper in Moscow at a price that will provide a high income?
Scrap metal collection points willingly accept unlimited quantities of products made from pure copper and its alloys, paying for them at favorable prices.
Our prices for copper intake
Type of medicinePrice per kg, rub
| Scrap copper glitter | 360-370 |
| A piece of copper | 350-360 |
| Copper mix | 335-345 |
| Scrap copper burner | 335-350 |
| Scrap tinned copper, burnt waste | 315-320 |
Specialists in the field can quickly and absolutely accurately determine the composition of scrap using spectral analysis using special devices - metal analyzers.
What can you do at home when options for determining the chemical composition of scrap metal are limited?
Some methods may be available to us, in particular:
- using one's own senses;
- use of readily available chemicals;
- exposure to fire;
- use of simple devices.
How to visually distinguish copper from other metals?
First of all, the material is carefully examined to determine the main external distinctive features of copper:
- the color of the metal should be golden pink;
- There is usually an oxide film on the surface, adding a yellowish-red tint.
Only a few metals have a similar color, including gold (Au), cesium (Cs), and osmium (Os). However, since these chemical elements are rare in their pure form and are produced in small quantities, it is almost impossible to make a mistake during visual inspection and identification of Cu among the listed materials.
The material should be viewed in good daylight, as artificial colors tend to be distorted. It is also recommended to get rid of the oxide film. This is done mechanically using a file, which is used to clean the surface, or identification is performed using a fresh cut.
Many copper products are marked. If you try to carefully examine the surface and find the markings, then its presence will allow you to accurately identify the material from the reference book.
How to distinguish copper from brass?
The situation is somewhat more complicated in identifying the copper alloy - brass. Brass scrap is accepted at collection points somewhat cheaper, since in addition to Cu it contains the chemical element zinc (Zn) in an amount of 4 to 45% of the alloy weight.
- Color. The color of the alloy is lighter than Cu. Moreover, the greater the amount of Zn it contains, the more the color turns yellow. Although in the case of a small amount of alloying additive (up to 10%), it is still difficult to identify the metal visually.
- Sound. Brass rings when struck, while soft copper produces a muffled sound. This method is good to use with fairly large products as an example.
- Plastic. When bending Cu, this occurs easily due to its high ductility. In the case of brass, this is much more difficult to do: the hardness of the alloy is higher and, accordingly, the flexibility is less.
- Weight. The average density of brass is 8.6 g/cu. cm, copper – 9 g/cu.m. see. You can identify metal using precise scales (if you have them in your house).
- Shape of chips. After processing, copper shavings have a spiral shape, and brass shavings have a straight needle shape.
- Chemical method. When exposed to hydrochloric acid, a brass alloy forms a white coating on its surface. If you do the same with copper, then nothing will happen.
Methods to distinguish copper from bronze
Simple methods that make it possible to find the differences between Cu and its alloy with tin (Sn):
- Plastic. If you apply pressure to the copper with something hard, a notch will appear in this place. It will not be possible to deform harder bronze in this way.
- Oxidation. Over time, the Cu surface becomes covered with an oxide film, which changes the color of the metal. Bronze alloy does not oxidize in air.
- Chemical method. In a metal container, a saline solution is heated to 50 degrees at the rate of 200 g of table salt per 1 liter of water. If you place copper there for 15 minutes, its color will change. Bronze alloy will not react in any way to such conditions.
How to distinguish from aluminum?
Tinned copper and copper-plated aluminum (Al) are often used in cable and wire products. Metals can be identified in this way:
- Color. Copper-plated aluminum has a yellow tint, and the surface of Cu after tinning becomes silver.
- Plastic. Copper conductors are several times more stable when bent and unbent, while aluminum conductors break quickly.
- Resistance measurement. The resistance of copper twisted pair (100 m) ranges from 4 to 8 Ohms, aluminum - from 12 to 20 Ohms.
- Exposure to flame. Al begins to melt at 600 degrees. The temperature at which signs of Cu melting appear is much higher.
how to distinguish copper from other metals
Any questions?
Order a consultation with a manager for free!
HOW TO DISTINGUISH COPPER FROM BRASS BY SOUND
It is advisable to apply this criterion to sufficiently large samples of copper and brass. Otherwise, the need to use auxiliary equipment cannot be avoided. When using sound perception, you need to know that a dull sound is characteristic of a low density, and a voiced sound is characteristic of a higher one:
- Copper samples, which are softer and have lower density, reproduce duller, low-quality sounds;
- Brass, unlike copper, produces a high, ringing sound with a tendency to increase.
DIFFERENCE IN HARDNESS
For this method of comparing copper and brass , suitable objects are suitable for bending and making dents:
- Copper, as a purer material, has greater ductility and the ability to deform without violating its integrity;
- Brass, which is an alloy of copper and reinforcing additives, has low ductility and higher strength and brittleness compared to copper, and therefore, if you try to deform, it will have greater resistance and may burst.
DIFFERENCE BETWEEN COPPER AND BRASS BY SHAVINGS
For such a comparison, you need to have a metal cutting machine or a drill with a large diameter attachment:
- Copper. It is distinguished by a florid shape, a long spiral without notches and good ductility (Figure 1);
- Brass. As a hard and brittle material, it produces crumbly, needle-like chips without a clear coil (Figure 2).
Figure 1. Copper shavings
Figure 2. Brass shavings
PRICE DIFFERENCE BETWEEN BRASS AND COPPER
The cost of pure metal is much more expensive than the alloy. Thus, the main differences between copper and brass can be displayed in the table:
| Copper | Brass |
| Plastic, soft | Solid |
| Reddish brown-pink shade | Golden tone |
| Lower sound on impact | Alt |
| Heavy | Easier |
| The shavings are twisted into a spiral | Needle shavings |