Tables of specific heat capacity of substances are presented: gases, metals, liquids, construction and thermal insulation materials, as well as food products - more than 400 substances and materials.
List of tables:
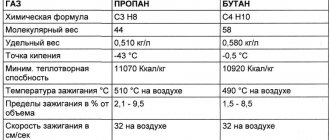
- Specific heat capacity of gases
- Specific heat capacity of some metals and alloys
- Specific heat capacity of liquids
- Specific heat capacity of solids
- Specific heat capacity of food
The specific heat capacity of a substance is the ratio of the amount of heat imparted to a unit mass of this substance in any process to the corresponding change in its temperature.
The specific heat capacity of substances depends on their chemical composition, thermodynamic state and the method of imparting heat to them. In the International System of Units, this value is measured in J/(kg K).
It should be noted that the experimental determination of the specific heat capacity of liquids and gases is carried out at constant pressure or at constant volume. In the first case, the specific heat capacity is denoted Cp , in the second - Cv . For liquids and gases, the most commonly used specific heat capacity at constant pressure is Cp.
For solids, the heat capacities Cp and Cv do not differ. In addition, in relation to solids, in addition to the specific mass heat capacity, specific atomic and molar heat capacities are also used.
Table of specific heat capacity of gases
The table shows the specific heat capacity of Cp gases at a temperature of 20°C and normal atmospheric pressure (101325 Pa).
Table of specific heat capacity of gases
| Gases | Cp, J/(kg K) |
| Nitrogen N2 | 1051 |
| Ammonia NH3 | 2244 |
| Argon Ar | 523 |
| Acetylene C2H2 | 1683 |
| Hydrogen H2 | 14270 |
| Air | 1005 |
| Helium He | 5296 |
| Oxygen O2 | 913 |
| Krypton Kr | 251 |
| Xenon Xe | 159 |
| Methane CH4 | 2483 |
| Neon Ne | 1038 |
| Nitric oxide N2O | 913 |
| Nitric oxide NO | 976 |
| Sulfur oxide SO2 | 625 |
| Carbon monoxide CO | 1043 |
| Propane C3H8 | 1863 |
| Hydrogen sulfide H2S | 1026 |
| Carbon dioxide CO2 | 837 |
| Chlorine Cl | 520 |
| Ethane C2H6 | 1729 |
| Ethylene C2H4 | 1528 |
Thermal conductivity of aluminum alloys - technical characteristics.
The thermal conductivity of aluminum is a technical parameter that characterizes the properties of the metal and alloys based on it. The value of this indicator is taken into account when forming compositions for the manufacture of foundry and deformable products, industrial production of parts and installations.
Thermal conductivity characteristics are taken into account when using it in production.
Characteristics of thermal conductivity of materials
The concept of thermal conductivity of materials is characterized by the ability to transfer thermal energy within a certain object from heated parts to cold ones. The process is carried out by atoms, molecules, electrons and occurs in any body with an uneven temperature distribution.
From the standpoint of kinetic physics, this process occurs as a result of the interaction of particles of molecules in hotter areas within the sample with other elements characterized by a lower temperature. The mechanism and rate of heat transfer depends on the state of aggregation of the substance.
The thermal conductivity category involves determining the heating rate of a material sample and the movement of a temperature wave in a certain direction. The indicator depends on physical parameters:
- density;
- temperature of phase transition to liquid state
- speed of sound propagation (for dielectrics).
The thermal conductivity coefficient is equal to the amount of heat that passes through a unit area of a homogeneous material per unit time with a temperature difference.
Physical properties of aluminum
The chemical element aluminum has a cubic crystal structure. Its specific gravity at 20 °C is 2.7 g/cm³, its melting point is +657...+660.2 °C, and its latent heat of fusion is 94.6 °C.
High purity aluminum boils at +1800…+2060 °C. When heated, the specific heat capacity of the metal, thermal conductivity and linear expansion coefficient increase.
The electrical conductivity of aluminum increases with decreasing temperature: at 189 °C it is 156 units, and at 400 °C it is 12.5.
Among chemical elements, aluminum is highly active. It easily reacts with oxygen, forming a dense oxide film that protects the metal from further influence of the environment.
The properties of an alloy are determined by the elements it contains.
As the temperature rises, hydrogen dissolves in the metal, increasing the porosity of the material. Impurities of alkaline chemical elements (potassium, sodium, calcium), silicon, magnesium contribute to a sharp increase in the porosity of aluminum.
Additives of copper, niobium, nickel, manganese, iron, chromium, vanadium, zirconium create a homogeneous structure when the molten material cools. The influence of alloy additions of other components on the physical properties of the metal and its alloys is taken into account in the technology of casting products.
The presence of additional materials changes the thermal conductivity of the composition and the melting point. For example, under normal conditions for the formation of aluminum alloys, sulfur and its compounds go into the slag without having a harmful effect on the properties of the composition.
Phosphorus, carbon, and nitrogen have the same effect. They do not change the mechanical properties of the alloy. Due to its reduced strength, pure aluminum is rarely used for the production of foundry products.
The lower the content of iron and silicon impurities, the higher the corrosion resistance of a metal. But their presence somewhat increases the strength of the material, while reducing ductility and electrical conductivity.
Technical characteristics of some aluminum-based alloys
According to technological features, alloys are divided into main groups:
- foundry - have enhanced foundry technological properties;
- deformable - easy to process under pressure.
For example, creating an aluminum structure used in construction requires a special type of alloy with increased strength that can withstand pressure and load.
Depending on the purpose of aluminum-based compositions, their formation is guided by norms and rules that take into account:
- heat conductivity of the material;
- point of transition from melt to solid state;
- the presence of ligature components that affect the technical parameters of the composition and increase strength.
The ratio of the main component to additives affects the thermal conductivity of the alloy, which is taken into account in the manufacture of radiators and other types of products intended for the installation of thermal communications.
Summary data on the thermal conductivity of aluminum alloys are collected in special reference books. They provide the values of common metal alloys with silicon, magnesium, copper, zinc, and duralumin. There are characteristics of casting alloys at various temperatures indicating the thermophysical properties of the composition. The main indicators are:
- density;
- thermal conductivity coefficient;
- coefficient of linear thermal expansion;
- strength change temperature;
- corrosion resistance in air;
- specific electrical resistance.
Analysis of the data indicates the dependence of the thermal conductivity coefficient on the increase in temperature and composition of the material. Low thermal conductivity is characteristic mainly of casting compositions based on aluminum with markings AK4, AL1, AL8.
The compositions of the main component with silicon and zinc have the highest density. Of the light materials, the most dense is the composition containing magnesium. The copper content in the material increases its strength and resistance to corrosion.
The densest alloys with zinc and magnesium
The higher the content of aluminum in the composition, the greater its thermal conductivity, which increases when the material is heated. The presence of lithium in the alloy composition leads to a decrease in the thermal conductivity coefficient.
The specific heat capacity of an alloy containing magnesium and silicon increases when heated. Among the aluminum alloys of the Al-Cu-Mn system, the most thermally conductive is the deformable composition D20.
It contains in small quantities (0.05–7%) impurities of iron, silicon, manganese, titanium, zirconium, magnesium, zinc and 91–93.5% aluminum and is intended for the manufacture of welded products operating at room or briefly elevated temperatures.
Similar articles
ometallah.com
Table of specific heat capacity of some metals and alloys
The table shows the specific heat capacity of some common metals and alloys at a temperature of 20°C.
You can find the heat capacity values of most metals at other temperatures in this table. Table of specific heat capacity of metals and alloys
| Metals and alloys | C, J/(kg K) |
| Aluminum Al | 897 |
| Aluminum bronze | 420 |
| Tin bronze | 380 |
| Tungsten W | 134 |
| Duralumin | 880 |
| Iron Fe | 452 |
| Au Gold | 129 |
| Constantan | 410 |
| Brass | 378 |
| Manganin | 420 |
| Copper Cu | 383 |
| Nickel Ni | 443 |
| Nichrome | 460 |
| Tin Sn | 228 |
| Platinum Pt | 133 |
| Mercury Hg | 139 |
| Lead Pb | 128 |
| Silver Ag | 235 |
| Reinforcing rod steel | 482 |
| Carbon steel | 468 |
| Chrome steel | 460 |
| Titan Ti | 520 |
| Uranium U | 116 |
| Zinc Zn | 385 |
| White cast iron | 540 |
| Gray cast iron | 470 |
Physical properties of metal
Aluminum is a chemical element (atomic number 13). It belongs to the group of light metals and is a common element found in the earth's crust.
Paramagnetic metal has a silvery-white color, it is very easy to machine, and it is convenient to cast products from it. The metal has high thermal and electrical conductivity. It is resistant to air due to the ability to form metal oxide films that protect the surface from the influence of the external environment.
The film is destroyed under the influence of alkaline solutions. To prevent the metal from reacting with aggressive liquids, indium, tin or gallium are added to the alloy.
The specific heat of fusion is 390 kJ/kg, and the specific heat of evaporation is 10.53 MJ/kg. The metal boils at a temperature of 2500°C. The melting gradient depends on the degree of purification of the material and is accordingly:
- for technical raw materials +658°C;
- for metal with highest class cleaning +660 °C.
Aluminum easily forms alloys, among which everyone knows compounds with copper, magnesium, and silicon. In the jewelry industry, this metal is combined with gold, which gives the composition new physical properties.
Aluminum easily forms alloys.
In nature, a chemical element forms natural compounds. It is found in minerals such as:
- nepheline;
- bauxite;
- corundum;
- feldspar;
- kaolinite;
- beryl;
- emerald;
- chrysoberyl.
In some places (volcano vents) native metal can be found in small quantities.
Table of specific heat capacity of liquids
The table shows the specific heat capacity Cp of common liquids at temperatures of 10...25°C and normal atmospheric pressure.
Table of specific heat capacity of liquids
| Liquids | Cp, J/(kg K) |
| Nitric acid (100%) NH3 | 1720 |
| Aniline C6H5NH2 | 2641 |
| Antifreeze (antifreeze) | 2990 |
| Acetone C3H6O | 2160 |
| Petrol | 2090 |
| Aviation gasoline B-70 | 2050 |
| Benzene C6H6 | 1050 |
| Water H2O | 4182 |
| Sea water | 3936 |
| Water is heavy D2O | 4208 |
| Vodka (40% vol.) | 3965 |
| Aqueous sodium chloride solution (25%) | 3300 |
| Gas oil | 1900 |
| Ammonium hydroxide | 4610 |
| Glycerol C3H5(OH)3 | 2430 |
| Dauterm | 1590 |
| Carborane C2H12B10 | 1720 |
| Kerosene | 2085…2220 |
| Kefir | 3770 |
| Fuel oil | 2180 |
| AMG-10 oil | 1840 |
| Oil VM-4 | 1480 |
| Castor oil | 2219 |
| Corn oil | 1733 |
| MS-20 oil | 2030 |
| Refined sunflower oil | 1775 |
| Oil TM-1 | 1640 |
| Transformer oil | 1680 |
| Refined cottonseed oil | 1737 |
| Oil HF-22 | 1640 |
| Condensed milk with sugar | 3936 |
| Whole milk | 3906 |
| Oil | 2100 |
| Liquid paraffin (at 50C) | 3000 |
| Beer | 3940 |
| Sulfuric acid (100%) H2SO4 | 1380 |
| Carbon disulfide CS2 | 1000 |
| Silicone | 2060 |
| Turpentine | 1800 |
| Cream (35% fat) | 3517 |
| Grape juice | 2800…3690 |
| Methyl alcohol (methanol) CH3OH | 2470 |
| Ethyl alcohol (ethanol) C2H5OH | 2470 |
| Whey | 4082 |
| Toluene C7H8 | 1130 |
| Diesel fuel (diesel fuel) | 2010 |
| Jet fuel | 2005 |
| Hexamine C6H12N4 | 1470 |
| Freon-12 CCl2F2 | 840 |
| Ethyl ether C4H10O | 2340 |
Specific Heat Capacity Table for Solids
The table shows the specific heat capacity of solid substances: building materials (sand, asphalt, etc.), thermal insulation of various types and other common materials in the temperature range from 0 to 50 ° C at normal atmospheric pressure.
Specific Heat Capacity Table for Solids
| Construction, thermal insulation and other materials | C, J/(kg K) |
| ABS plastic | 1300…2300 |
| Aggloporite concrete and concrete based on fuel (boiler) slags | 840 |
| Diamond | 502 |
| Argillite | 700…1000 |
| Fibrous asbestos | 1050 |
| Asbestos cement | 1500 |
| Asbotekstolite | 1670 |
| Asboshifer | 837 |
| Asphalt | 920…2100 |
| Asphalt concrete | 1680 |
| Airgel (Aspen aerogels) | 700 |
| Basalt | 850…920 |
| Barite | 461 |
| Birch | 1250 |
| Concrete | 710…1130 |
| Bitumen perlite | 1130 |
| Petroleum bitumens for construction and roofing | 1680 |
| Paper | 1090…1500 |
| Mineral wool | 920 |
| Glass wool | 800 |
| Cotton wool | 1675 |
| Slag wool | 750 |
| Vermiculite | 840 |
| Vermiculite concrete | 840 |
| Viniplast | 1000 |
| Woolen felt | 1700 |
| Wax | 2930 |
| Gas and foam concrete, gas and foam silicate, gas and foam ash concrete | 840 |
| Getinax | 1400 |
| Dry molded gypsum | 1050 |
| Drywall | 950 |
| Clay | 750 |
| Fireproof clay | 800 |
| Alumina | 700…840 |
| Gneiss (facing) | 880 |
| Gravel (filler) | 850 |
| Expanded clay gravel | 840 |
| Shungizite gravel | 840 |
| Granite (cladding) | 880…920 |
| Graphite | 708 |
| Wet ground (soil) | 2010 |
| Lunar soil | 740 |
| Sandy soil | 900 |
| The soil is dry | 850 |
| Tar | 1675 |
| Diabase | 800…900 |
| Dinas | 737 |
| Dolomite | 600…1500 |
| Oak | 2300 |
| Reinforced concrete | 840 |
| Reinforced concrete | 840 |
| Wood ash | 750 |
| Limestone (cladding) | 850…920 |
| Products made from expanded perlite with a bitumen binder | 1680 |
| Sandy silt | 1000…2100 |
| Building stone | 920 |
| Capron | 2300 |
| Carbolite black | 1900 |
| Corrugated cardboard | 1150 |
| Cardboard facing | 2300 |
| Thick cardboard | 1200 |
| Multilayer construction cardboard | 2390 |
| Natural rubber | 1400 |
| Crystalline quartz | 836 |
| Quartzite | 700…1300 |
| Expanded clay | 750 |
| Expanded clay concrete and expanded clay foam concrete | 840 |
| Dinas brick | 905 |
| Carborundum brick | 700 |
| Red dense brick | 840…880 |
| Magnesite brick | 1055 |
| Facing brick | 880 |
| Fireproof semi-acid brick | 885 |
| Silicate brick | 750…840 |
| Construction brick | 800 |
| Treble brick | 710 |
| Fireclay brick | 930 |
| Masonry "Poroton" | 900 |
| Rubble masonry made of medium-density stones | 880 |
| Gas silicate masonry | 880 |
| Masonry made of ordinary clay bricks | 880 |
| Ceramic hollow brick masonry | 880 |
| Sand-lime brick masonry | 880 |
| Treble brick masonry | 880 |
| Slag brick masonry | 880 |
| Powdered coke | 1210 |
| Corundum | 711 |
| Oil paint (enamel) | 650…2000 |
| Silicon | 714 |
| Volcanic lava | 840 |
| Brass | 400 |
| Ice from heavy water | 2220 |
| Ice at 0°C | 2150 |
| Ice at -100°C | 1170 |
| Ice at -20°C | 1950 |
| Ice at -60°C | 1700 |
| Linoleum | 1470 |
| Asbestos-cement flat sheets | 840 |
| Gypsum cladding sheets (dry plaster) | 840 |
| Sunflower husk | 1500 |
| Magnetite | 586 |
| Malachite | 740 |
| Stitched fiberglass mats and strips | 840 |
| Mineral wool mats, stitched and with a synthetic binder | 840 |
| Chalk | 800…880 |
| Mikanite | 250 |
| Mipora | 1420 |
| Marble (cladding) | 880 |
| Deck flooring | 1100 |
| Naphthalene | 1300 |
| Nylon | 1600 |
| Neoprene | 1700 |
| Tow | 2300 |
| Paraffin | 2890 |
| Oak parquet | 1100 |
| Piece parquet | 880 |
| Panel parquet | 880 |
| Pumice concrete | 840 |
| Foam concrete | 840 |
| Foam plastic PVC-1 and PV-1 | 1260 |
| Expanded polystyrene | 1340 |
| Expanded polystyrene "Penoplex" | 1600 |
| Polyurethane foam | 1470 |
| Foam glass or gas glass | 840 |
| Glassine | 1680 |
| Reinforced ceramic ceiling with concrete filling without plaster | 850 |
| Flooring made of reinforced concrete elements with plaster | 860 |
| Monolithic flat reinforced concrete floor | 840 |
| Perlite concrete | 840 |
| Perlitoplast-concrete | 1050 |
| Perlite phosphogel products | 1050 |
| Sand for construction work | 840 |
| Fine river sand | 700…840 |
| Fine river sand (wet) | 2090 |
| Sand sugar | 1260 |
| Sand dry | 800 |
| Fir | 2700 |
| Polyester plastic | 1000…2300 |
| Cork plate | 1850 |
| Alabaster slabs | 750 |
| Wood-fiber and particle boards (chipboard, fiberboard) | 2300 |
| Gypsum slabs | 840 |
| Resol-formaldehyde foam boards | 1680 |
| Glass staple fiber boards with synthetic binder | 840 |
| Reed slabs | 2300 |
| Flax insulating slabs | 2300 |
| High-hardness mineral wool slabs | 840 |
| Semi-rigid mineral wool slabs with starch binder | 840 |
| Peat thermal insulation slabs | 2300 |
| Fiberboard and wood concrete slabs based on Portland cement | 2300 |
| Carpet covering | 1100 |
| Seamless gypsum floor | 800 |
| Polyvinyl chloride (PVC) | 920…1200 |
| Polycarbonate (Diflon) | 1100…1120 |
| Polymethyl methacrylate | 1200…1650 |
| Polypropylene | 1930 |
| Polystyrene UPP1, PPS | 900 |
| Polystyrene concrete | 1060 |
| Polyvinyl chloride | 1130…1200 |
| Polychlorotrifluoroethylene | 920 |
| High Density Polyethylene | 1900…2300 |
| Low density polyethylene | 1700 |
| Portland cement | 1130 |
| Cork | 2050 |
| Cork granulated | 1800 |
| Gypsum grout mortar | 900 |
| Gypsum perlite solution | 840 |
| Porous gypsum perlite solution | 840 |
| Lime-sand mortar | 840 |
| Lime mortar | 920 |
| Complex mortar (sand, lime, cement) | 840 |
| Cement-perlite mortar | 840 |
| Cement-sand mortar | 840 |
| Cement-slag mortar | 840 |
| Soft rubber | 1380 |
| Porous rubber | 2050 |
| Ordinary hard rubber | 1350…1400 |
| Ruberoid | 1500…1680 |
| Sulfur | 715 |
| Slate | 700…1600 |
| Mica | 880 |
| Epoxy resin | 800…1100 |
| Stale snow at 0°C | 2100 |
| Freshly fallen snow | 2090 |
| Pine and spruce | 2300 |
| Resinous pine 15% humidity | 2700 |
| Mirror glass (mirror) | 780 |
| Quartz glass | 890 |
| Laboratory glass | 840 |
| Ordinary glass, window | 670 |
| Flint glass | 490 |
| Glass wool | 800 |
| Fiberglass | 840 |
| Fiberglass | 800 |
| Pressed wood shavings | 1080 |
| Textolite | 1470…1510 |
| Tol | 1680 |
| Peat | 1880 |
| Peat slabs | 2100 |
| Tuff (facing) | 750…880 |
| Tufobeton | 840 |
| Charcoal | 960 |
| Coal | 1310 |
| Plywood | 2300…2500 |
| Porcelain | 750…1090 |
| Fibrolite (gray) | 1670 |
| Zircon | 670 |
| Chamotte | 825 |
| Slate | 750 |
| Granulated slag | 750 |
| Boiler slag | 700…750 |
| Cinder concrete | 800 |
| Slag pumice concrete (thermosite concrete) | 840 |
| Slag pumice foam and slag pumice gas concrete | 840 |
| Gypsum plaster | 840 |
| Polystyrene mortar plaster | 1200 |
| Lime plaster | 950 |
| Lime plaster with stone dust | 920 |
| Perlite plaster | 1130 |
| Facade plaster with polymer additives | 880 |
| Shungizite concrete | 840 |
| Crushed stone and sand from expanded perlite | 840 |
| Crushed stone from blast furnace slag, slag pumice and agloporite | 840 |
| Ebonite | 1430 |
| Ecowool | 2300 |
| Etrol | 1500…1800 |
Edgar Allan Poe
- American writer, poet, literary critic and editor, is a representative of American romanticism, the forerunner of symbolism and decadence. He received the greatest fame for his “dark” stories (he was one of the first American writers to create his works in the form of short stories). Creator of the modern detective form. The work of Edgar Allan Poe contributed to the emergence of the science fiction genre.
Edgar Allan Poe, one of the greatest American romantics of the 19th century, was born on January 19, 1809 in Boston.
His father abandoned his family, and his mother died of a serious illness
when little Edgar was not even three years old.
The child was taken into care by the family of a wealthy merchant from Richmond, John Allan
, who after some time moved to England, where the boy was sent to study at a prestigious boarding school.
Edgar developed early: at the age of five he read, drew, wrote, recited, and rode horseback. At school he studied well, acquired a large stock of knowledge in literature, especially English and Latin, in general history, in mathematics, in some branches of natural science, such as astronomy and physics. Physically, Edgar was strong, participated in all the pranks of his comrades, and at the university - in all their revelry. The character of the future poet from childhood was uneven, passionate, impetuous. Many strange things were noted in his behavior. From an early age, Edgar wrote poetry, was fond of fantastic plans, and loved to carry out psychological experiments on himself and others. Realizing his superiority, he made others feel it.
In 1820, the Allan family returned to Richmond, where Edgar attended college. Soon, he incurred large gambling debts. A quarrel with the Allans ensued, and Poe left their home. Shortly before this, he suffered a brain injury that resulted in periodic nervous seizures. In 1827, Poe returned, possibly via Baltimore, to Boston, where his collection Tamerlane and Other Poems was published early in the summer. That same year he enlisted in the army and was sent to the fort.
In the period from 1833 to 1840, the author published many poems and stories, and worked for the Southern Literary Messenger magazines in Richmond. From 1841 to 1843 he lived with his family in the suburbs of Philadelphia and worked for Burton's Gentleman's Magazine and Graham's Magazine. In Philadelphia, Edgar Poe also intended to publish his own magazine, The Stylus (or The Penn), but this venture failed.
E. Poe married Virginia Clemm in 1835. He was 27, she was 13.
In 1846, the New York magazine with which he collaborated, the Broadway Journal, closed, and Poe lost his livelihood. Disastrous life resumed.
In 1838, Edgar accepted an offer to take the position of editor at Gentelmen's magazine and moved to Philadelphia because of this. In 1839, he accumulated enough wealth to publish the book “Grotesques and Arabesques.” The writer lived in Philadelphia for six years, during which time he published about thirty stories and many literary critical articles.
In 1844, Edgar returned to New York and published several short stories there, but they were not successful with the public, but the poem “The Raven”, published in 1845, and the collection of the same name made Poe incredibly popular. But soon the bright streak of life ended, poverty came again. In the same year, his beloved wife Virginia died from a long illness (tuberculosis).
From grief and hopelessness, the writer completely loses his head, drinks a lot, starts using drugs to brighten up his loneliness, he visits prostitutes more and more often, and during the next binge he even tries to commit suicide. At this time, his book “Eureka” was published - he considered it “the greatest revelation that humanity has ever heard,” but the work did not find a response in the hearts of “humanity.”
The last years of Edgar Poe's life, 1847-1849,
were years of tossing, half-madness, successes, falls and constant slander. Virginia, dying, took an oath from Mrs. Shew, Edgar's friend, never to leave him. Edgar Poe was captivated by women, imagined that he was in love, and there was even talk of marriage. In life, he behaved strangely, but managed to publish several more brilliant works.
But Edgar Allan Poe’s attacks of alcoholism became more and more painful, his nervousness increased almost to the point of mental disorder. Mrs. Shew felt it necessary to remove herself from his life. In the autumn of 1849 the end came. Full of chimerical projects, considering himself a bridegroom again, Edgar Allan Poe gave a lecture on the “Poetic Principle” in Richmond in September of this year with great success. Edgar Poe left Richmond with $1,500 in his pocket. What happened next remains a mystery. Apparently, he was drugged by robbers: Edgar Allan Poe was found unconscious, completely naked, and robbed. He was taken to a hospital in Baltimore, where he died on October 7, 1849.
Information sources:
- ru.wikipedia.org - Wikipedia: Edgar Allan PO;
- 5ballov.qip.ru - biography of Edgar Allan Poe;
- fantlab.ru - biography of Edgar Allan Poe.
Additionally on Guenon:
- Who created the first detective stories?