Scientific point of view
To determine which metals are not magnetic, you need to find out how all metals in general can relate to magnets and a magnetic field. With respect to the applied magnetic field, all substances are divided into diamagnetic, paramagnetic and ferromagnetic.
Each atom consists of a positively charged nucleus and negatively charged electrons. They move continuously, which creates a magnetic field. The magnetic fields of electrons in one atom can enhance or cancel each other, depending on the direction of their movement. Moreover, the following can be compensated:
- Magnetic moments caused by the movement of electrons relative to the nucleus are orbital.
- Magnetic moments caused by the rotation of electrons around their axis are spin moments.
If all magnetic moments are equal to zero, the substance is classified as diamagnetic. If only spin moments are compensated - to paramagnets. If the fields are not compensated, use ferromagnets.
Application of ferromagnets, examples
Ferromagnetic substances, due to their special physical and chemical properties, have found wide application in various fields of electrical engineering. Using soft magnetic types of ferromagnets, equipment and units are produced such as:
- transformers;
- electric motors;
- generators;
- low-current communication technology;
- radio engineering.
Ferromagnets, in the absence of an external magnetic field, remain magnetized, creating a magnetic field in the external environment. Elementary currents in matter retain an ordered orientation. This property is actively used in modern industry to create permanent magnets, which are used to manufacture the following types of equipment:
- electrical measuring instruments;
- loudspeakers;
- telephones;
- sound recording equipment;
- magnetic compasses.
Materials related to ferrites, which have both ferromagnetic and semiconductor properties, are widespread in the production of radio engineering. The substances are actively used in the manufacture of inductor cores, magnetic tapes, films and disks.
Paramagnets and ferromagnets
Let's consider the option when each atom of a substance has its own magnetic field. These fields are multidirectional and compensate each other. If you place a magnet next to such a substance, the fields will be oriented in one direction. The substance will have a magnetic field, a positive and a negative pole. Then the substance will be attracted to the magnet and can itself become magnetized, that is, it will attract other metal objects. For example, you can magnetize steel clips at home. Each one will have a negative and a positive pole, and you can even hang a whole chain of paper clips on a magnet. Such substances are called paramagnetic.
Ferromagnets are a small group of substances that are attracted to magnets and are easily magnetized even in a weak field.
Diamagnets
In diamagnetic materials, the magnetic fields inside each atom are compensated. In this case, when a substance is introduced into a magnetic field, the movement of electrons under the influence of the field will be added to the natural movement of electrons. This movement of electrons will cause an additional current, the magnetic field of which will be directed against the external field. Therefore, the diamagnetic material will be weakly repelled from the nearby magnet.
So, if we approach the question from a scientific point of view, which metals are not magnetic, the answer will be – diamagnetic.
How to demagnetize metal at home
Craftsmen when working with various metals are faced with a problem - magnetization of tools. In some jobs, magnetic properties help with actions, for example, with a magnetic screwdriver you can install a screw in a hard-to-reach place. The build-up of metal shavings when using a caliper, file or drill can interfere with marking or a straight cut line.
The main reasons for metal magnetization
Magnets are media that create their own magnetic field. Main groups of magnets:
- paramagnetic materials;
- ferromagnets;
- Diamagnets.
Steel products based on alloys of iron, cobalt or nickel refer to substances whose own magnetic field is higher than the external one, i.e. to ferromagnets. The magnetization of a substance is considered to be the sum of the magnetic properties of particles per unit volume.
At the moment the Curie temperature threshold is reached, spontaneous domains with magnetization are formed, which spread until they are completely filled. Under normal conditions, it is possible to obtain a magnetized tool when working close to electric motors, magnetrons and other elements. The metal takes away the properties of magnetism from a nearby emitter, thereby becoming magnetized.
Working with small parts with a magnetized tool can cause a lot of trouble. Sharpening metals with increased magnetism properties is impossible to ideal sizes, because... the material is covered with shavings.
Using a demagnetization device
The demagnetization device comes in three variations. The basic elements can be selected at home, using simple methods that do not require much effort to manufacture. There are special devices that can both demagnetize and magnetize an element.
Magnetometers are used in the following sequence:
- The magnetic field strength of the instrument is an important parameter that needs to be determined, because it is possible to get a negative result;
- the same parameter must be found on a magnet of the opposite sign;
- touching the tool to the area of the device will demagnetize it.
The process takes place within 10 seconds; at home, no connection to the electrical network is required. The functionality is checked as follows: the self-tapping screw is brought to the magnetized metal, and the level of magnetization is checked. Afterwards the demagnetization process occurs and is checked again.
Methods for demagnetizing metal
There are several ways to demagnetize metal structures. Devices are used depending on the frequency of use, purpose and power. Before you demagnetize metal at home, you need to understand the existing structures.
- An ordinary magnet is large in size; a tool is held over it at a minimum distance, on the verge of the attraction process. The magnet can be removed from an old speaker, most of which are round in shape. The process is carried out when the product is removed from the structure, loosening it; the further the tool is from the structure, the smaller the amplitude. The location of the axis on which there is no magnetic field depends on the design of the product.
- More frequent use will require a device operated at home from the mains. It is possible to make the device at home or purchase it at radio parts stores. The main component is a coil of wound wire connected to a transformer. The supply of alternating current allows you to demagnetize the element, direct current - vice versa.
Removing magnetization with a magnetometer
There are many variations and kits for demagnetizing metals in production.
Tunnel devices include a coil having an opening connected to a network.
The size of the hole can be different, depending on the purpose and dimensions of the parts being processed. Multi-band magnets driven by motion, rotation of which occurs with speed control, the impact and change in amplitude is carried out by moving the part away from the body.
Electromagnets operate from a 220 or 380 volt network and allow you to demagnetize an element with a tap for a certain time. Container mechanisms allow you to install the product to a device in which the necessary environment is automatically created.
How to make a demagnetization device at home
It is possible to make an electromagnet for demagnetization at home; for this you will need some materials and available tools. Operation occurs by controlling the current; constant voltage can magnetize the element, while alternating voltage, on the contrary, produces action.
Homemade device for demagnetizing metals
The coil can be made from parts of an old TV, or rather the demagnetization loop of a kinescope. It is important to maintain consistency during manufacturing for a correct process.
- The loop is folded several times until the coil reaches the required diameter. If one loop is not enough, you can sequentially add a second one; this design will allow you to work with large elements.
- A fuse and a button are connected for normal, uninterrupted operation.
- Designs for 220 Volts can be used constantly, those designed for 110 V are connected for a short time, 12 V are used through a transformer.
Installation for demagnetization from a transformer
The resulting mechanism is perfect for large parts. When working with small devices, you can prepare a mini kit at home. For operation, any coil is used, for example from an old reel-to-reel player, connected in series with a transformer. Use occurs by applying voltage, the part is placed near the mechanism, then removed, while the power of the device remains on.
, please select a piece of text and press Ctrl+Enter.
Homemade demagnetizer or how to demagnetize a tool
Sometimes a magnetized tool is useful - for example a screwdriver, the screw will not fall off. And when a file, tap, drill, or pliers are magnetized, this is not very good, rather even very bad in terms of the adhesion of metal filings and their subsequent removal. This article will discuss the topic of how you can make a demagnetizer with your own hands and using improvised means.
Distribution of paramagnets and diamagnets in the periodic table of Mendeleev elements
The magnetic properties of simple substances change periodically with increasing atomic number of the element.
Substances that are not attracted to magnets (diamagnets) are located mainly in short periods - 1, 2, 3. Which metals are not magnetic? These are lithium and beryllium, and sodium, magnesium and aluminum are already classified as paramagnetic.
Substances that are attracted to magnets (paramagnets) are located mainly in the long periods of the Mendeleev periodic system - 4, 5, 6, 7.
However, the last 8 elements in each long period are also diamagnetic.
In addition, three elements are distinguished - carbon, oxygen and tin, the magnetic properties of which are different for different allotropic modifications.
In addition, there are 25 more chemical elements whose magnetic properties could not be established due to their radioactivity and rapid decay or the complexity of synthesis.
The magnetic properties of lanthanides and actinides (all of which are metals) change irregularly. Among them there are para- and diamagnetic materials.
There are special magnetically ordered substances - chromium, manganese, iron, cobalt, nickel, the properties of which change irregularly.
What metals are not magnetic: list
There are only 9 ferromagnets, that is, metals that are highly magnetic, in nature. These are iron, cobalt, nickel, their alloys and compounds, as well as six lanthanide metals: gadolinium, terbium, dysprosium, holmium, erbium and thulium.
Metals that are attracted only to very strong magnets (paramagnetic): aluminum, copper, platinum, uranium.
Since in everyday life there are no such large magnets that would attract a paramagnetic material, and also no lanthanide metals are found, we can safely say that all metals except iron, cobalt, nickel and their alloys will not be attracted to magnets.
So, what metals are not magnetic to a magnet:
- paramagnetic materials: aluminum, platinum, chromium, magnesium, tungsten;
- diamagnetic materials: copper, gold, silver, zinc, mercury, cadmium, zirconium.
In general, we can say that ferrous metals are attracted to a magnet, non-ferrous metals are not.
If we talk about alloys, then iron alloys are magnetic. These primarily include steel and cast iron. Precious coins can also be attracted to a magnet, since they are not made of pure non-ferrous metal, but of an alloy that may contain a small amount of ferromagnetic material. But jewelry made of pure non-ferrous metal will not be attracted to a magnet.
Steels and alloys with magnetic and electrical properties
Steels and alloys with magnetic properties. Magnetic steels and alloys are divided into two groups: hard magnetic and soft magnetic.
Hard magnetic
steels and alloys have a high coercive force
Hc
and residual induction
Br.
They are used to make permanent magnets. Small permanent magnets are made from carbon hypereutectoid steels UYU-U12.
The coercive force of carbon steels increases sharply after hardening to martensite due to the appearance of high stresses.
For steel U12 after quenching in water Hs
= 4800 A/m,
Br
= 0.8 T. However, low hardenability and low stability of residual induction have led to the displacement of carbon steels by alloy steels.
Alloying a metal causes an increase in magnetic hardness (i.e., coercive force). The coercive force increases with the formation of a second phase in a solid solution, with increasing discreteness of the second phase, with the occurrence of stresses in the crystal lattice, and with grain refinement.
Currently, for the manufacture of permanent magnets, steels alloyed with chromium, tungsten, cobalt or several elements together (EX3, EX7B6, EX5K5) are widely used. The letter E stands for magnetic steel.
To obtain high magnetic properties, steel is subjected to complex heat treatment, consisting of normalization, quenching in oil or water, and low-temperature tempering (at 100°C for 10-24 hours).
The high content of carbon and alloying elements in these steels gives them increased hardness, so before cold machining they are subjected to softening annealing at 700-850 °C. During annealing, carbides are formed, which impairs the magnetic properties (“magnetic damage”). Therefore, before hardening, to eliminate “magnetic damage,” normalization is carried out, during which large carbide phases dissolve.
To avoid “magnetic damage” during hardening, heating should be short-term (no more than 15 minutes). Cooling can be done in water or oil, but is usually cooled in oil to avoid warping and cracking, although this does reduce the magnetic properties somewhat.
Cold treatment increases magnetic properties as it eliminates non-magnetic (paramagnetic) austenite.
Tempering somewhat reduces the coercive force, but ensures the stability of the magnetic properties during operation.
Iron-nickel-cobalt alloys, in particular magnet (8% Al, 24% Co, 14% Ni, 3% Cu, the rest iron), have high magnetic properties.
Magnets from this alloy are produced by casting, since the alloy cannot be deformed or processed by cutting. The alloy is quenched in a magnetic field. The essence of hardening is as follows. The alloy heated to 1300°C is placed between the poles of an electromagnet with a voltage of 160 A/m and cooled to a temperature below 500°C, further cooling is carried out in air. After such treatment, the alloy has anisotropy of magnetic properties.
Magnetic properties reach a high level in the direction in which the external magnetic field acted during quenching. Then the alloy is tempered at 600 °C. Magnetic properties: I = 40,000 A/m, Br
= 1.2 T.
Recently, alloys based on cobalt (52% Co, 14% V, the rest iron) are being used. The alloy is supplied in the form of tapes, strips, etc.
Soft magnetic
alloys and steels have low coercivity and high magnetic permeability. They are used for the manufacture of cores and magnetic devices operating in alternating magnetic fields. Soft magnetic materials must have a uniform (homogeneous) structure and large grain.
A slight cold hardening greatly reduces the magnetic permeability and increases the coercive force. Therefore, soft magnetic alloys are subjected to recrystallization annealing to relieve stress and distortion of the structure.
Pure iron, in which the content of carbon and all impurities is strictly limited, is widely used. Iron is used for the manufacture of relay cores, DC electromagnets, poles of electrical machines, etc.
Electrical steel has found wide application in industry
- an alloy of iron and silicon (0.05-0.005% C, 1.0-1.8% Si). Alloying with silicon increases the electrical resistance of steel and thereby reduces eddy current losses, increases magnetic permeability, reduces coercive force and hysteresis losses, promotes grain growth, and improves magnetic properties due to the graphitizing effect.
Electrical steels are marked as follows: the first digit indicates the type of rolled product and structural state (1 - hot-rolled, 2 - cold-rolled isotropic, 3 - cold-rolled anisotropic); second - silicon content: 0 - up to 0.4%; 1 - 0.4-0.8%; 2 - 0.8-1.8%; 3 - 1.8-2.8%; 4 - 2.8-3.8%; 5 - 3.8-4.8%; the third is the main normalized characteristic (0, 1 and 2 are specific losses at different values of magnetic induction and frequency, 6 and 7 are magnetic induction in weak and medium fields, respectively). Together, the first three digits indicate the type of steel; the fourth is the serial number of the steel type. The higher it is, the lower the specific losses, the greater the magnetic induction.
To remove hardening after rolling and to enlarge the grain, electrical steel is annealed at 1100-1200 °C in a hydrogen atmosphere.
When cutting sheets, cutting, stamping, bending, the magnetic properties deteriorate. To restore the magnetic properties of electrical steel, it is recommended to anneal at 750–800 °C for 2 hours with slow (-50 deg/h) cooling to 400 °C.
In this case, it is necessary to exclude oxidation and carburization of steel.
Electrical steel is manufactured in the form of sheets with a thickness of 1 to 0.05 mm.
Iron-nickel alloys
(from 40 to 80% Ni) - permalloy - have high magnetic permeability, which is very important for devices operating in weak fields (radio, telephone, telegraph). The magnetic properties of permalloy are highly dependent on heat treatment.
To improve the magnetic properties after mechanical treatment, permalloy is annealed at 1100-1200 °C in a vacuum or hydrogen atmosphere. At the same time, the grain is enlarged, residual stresses are eliminated and carbon impurities are removed.
Cooling in a magnetic field also leads to an increase in magnetic properties.
Non-magnetic steels.
In electrical engineering and instrument making, many parts are made from non-magnetic steels. Previously, non-ferrous metals were used for this purpose, but now non-magnetic austenitic steels are widely used. The use of these steels dramatically reduces the cost of parts, and also increases mechanical properties and reduces eddy current losses in electrical equipment.
The use of manganese austenitic wear-resistant steel (11OG13L) as a non-magnetic steel is limited by its poor cutting machinability, which is due to its high tendency to work hardening, as well as the instability of strength properties.
Austenitic corrosion-resistant steels 12Х18Н9, 12Х18Н9Т are widely used. It is desirable that the nickel content in them corresponds to the upper limit, since otherwise, at high degrees of cold deformation, a partial occurrence of γ→α is possible - a transformation leading to the appearance of ferrite with ferromagnetic properties.
In addition, cheaper steels 55G9N9KhZ and 45G17YUZ are used, in which nickel is partially or completely replaced by manganese.
Steels and alloys with electrical properties . Electrical resistance elements must have low electrical conductivity or high electrical resistance. Since the formation of solid solutions during alloying is accompanied by an increase in electrical resistance, all high-resistance alloys, as a rule, are solid solutions.
rheostatic alloys
(for the manufacture of rheostats) and scale-resistant alloys
of high electrical resistance
(for heating elements of furnaces and electrical appliances).
Alloys of high electrical resistance must meet the following requirements:
have a high electrical resistivity;
have a low temperature coefficient of electrical resistance (i.e., electrical resistance should change little with temperature changes);
have high scale resistance, i.e. ability to resist scale formation at high temperatures.
Alloys of copper and nickel - constantan and nickel - have found widespread use as rheostatic alloys. Constantan contains 40% Ni, 1-2% Mn, the rest is copper; nickel - 45% Ni, the rest copper.
As alloys of high electrical resistance, the alloys used are Ni - Cr (nichromes), Fe - Ni - Cr (ferronichromes) and Fe - Cr - Al (fechral), etc.
The properties of alloys of high electrical resistance are adversely affected by impurities such as carbon, sulfur, phosphorus, etc. Impurities promote oxidation of grain boundaries and thereby reduce oxidation and increase brittleness.
In instrument making, alloys with a certain coefficient of linear expansion, for example, the same as that of glass, equal to zero, are often required. To meet these requirements, alloys of strictly defined composition are manufactured in each specific case.
Wear-resistant steels. Wear of parts during operation can be caused by two reasons: friction of parts against each other and scratching of solid particles on the surface of parts (abrasive wear).
With normal friction, the metal surface becomes hardened and wear resistance increases. Consequently, wear resistance is determined by the ability of the metal to work harden.
In the case of abrasive wear, when hard particles, abrasives, tear out tiny pieces of metal, wear resistance is determined by the metal's tear resistance and hardness.
For the manufacture of parts subject to wear under conditions of friction and high pressures and impacts, high-manganese austenitic steel 110G13L is used, containing 1.0-1.3% C and 11.5-14.5% Mn. Steel is used in cast and less often in hot-deformed state. The structure of cast steel consists of austenite and excess carbides (Fe, Mn)3C, which precipitate along the grain boundaries and reduce the strength and toughness of the steel. To increase strength and toughness, steel is hardened at a temperature of 1050–1100°C in water. At this temperature, carbides dissolve, and rapid cooling in water completely delays their release. After hardening, the steel has an austenitic structure and has the following mechanical properties: σв= 800-900 MPa, σ0.2 = 310…350 MPa, δ=15…25%, ψ= 20…30%, 180…220 НВ.
The high wear resistance of 110G13L steel during friction with pressure and impacts is explained by its increased ability to work harden.
If during operation only abrasive wear is observed without significant pressure and impacts causing hardening, then the steel does not exhibit increased wear resistance.
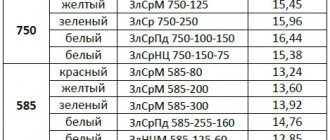
Table 8
Alkali metals are the MOST DANGEROUS and Active Elements! (December 2019).
Magnets are materials that create magnetic fields that attract certain metals. Every magnet has a north and south pole. Reverse poles attract while poles repel.
While most magnets are made from metals and metal alloys, scientists have developed ways to create magnets from composite materials such as magnetic polymers.
What creates magnetism?
Magnetism in metals is created by the uneven distribution of electrons in the atoms of certain metallic elements.
The uneven rotation and motion caused by this uneven distribution of electrons shifts the charge within the atom back and forth, creating magnetic dipoles.
When magnetic dipoles align, they create a magnetic domain, a localized magnetic region with north and south poles.
In non-magnetic materials, magnetic domains collide in different directions, canceling each other out. Whereas in magnetized materials, most of these domains are aligned, pointing in the same direction, which creates a magnetic field. The more areas that line up with each other, the stronger the magnetic force.
Magnet types:
- Permanent magnets (also known as hard magnets) are those that constantly produce a magnetic field. This magnetic field is caused by ferromagnetism and is the strongest form of magnetism.
- Temporary magnets (also known as soft magnets) are only magnetic in the presence of a magnetic field.
- Electromagnets require electric current to pass through their coil wires to create a magnetic field.
Development of magnets:
Greek, Indian and Chinese writers documented basic knowledge of magnetism more than 2,000 years ago. Much of this understanding was based on observations of the effects of magnesium (the naturally occurring magnetic mineral of iron) on iron.
Early research into magnetism dates back to the 16th century, but the development of modern high-strength magnets did not occur until the 20th century.
Before 1940, permanent magnets were used only in basic applications such as compasses and electrical generators called magnetos. The development of aluminum and nickel-cobalt (Alnico) magnets has allowed permanent magnets to replace electromagnets in motors, generators and loudspeakers.
The development of samarium-cobalt (SmCo) magnets in the 1970s created magnets with twice the magnetic energy density of any previously available magnet. Smaller, more powerful magnets contributed to the development of many electronic devices as we know them.
By the early 1980s, further research into the magnetic properties of rare earth elements led to the discovery of neodymium-iron-boron (NdFeB) magnets. NdFeB magnets again led to a doubling of magnetic energy over SmCo magnets.
Rare earth magnets are now used in everything from wristwatches and iPads to hybrid car engines and wind turbines.
Magnetism and temperature:
Metals and other materials have different magnetic phases, depending on the temperature of the environment in which they are located. As a result, a metal can exhibit more than one form of magnetism.
Iron, for example, loses its magnetism, becoming paramagnetic when heated above 1418 °F (770 °C).
The temperature at which a metal loses its magnetic force is called its Curie temperature.
Iron, cobalt and nickel are the only elements that, in metallic form, have Curie temperatures above room temperature. Thus, all magnetic materials must contain one of these elements.
Common ferromagnetic metals and their curie temperatures:
| Substance | Curie temperature |
| Iron (Fe) | 1418 °F (770 °C) |
| Cobalt (Co) | 2066 °F (1130 °C) |
| Nickel (Ni) | 676.4°F (358°C) |
| Gadolinium | 66°F (19°C) |
| Dysprosium | -301. 27°F (-185.15°C) |
Sources: How Stuff Works, Inc. How do magnets work? //science. How it works. com/magnet1. HTM Wikipedia. Curie temperature. // ru. Wikipedia. org/wiki/Curie_temperature
Loss of ferromagnetism properties
Ferromagnetic substances are called “magnetically frozen” paramagnets. Atoms of paramagnetic materials have magnetic moments that are in chaotic rotational motion. In the case of ferromagnets, the moments are definitely directed. As the temperature increases, the number of random temperature fluctuations of the magnetic moments of atoms increases. If the temperature of the ferromagnetic becomes close to the Curie temperature, that is, comparable to the magnetic “melting” temperature, the ferromagnetic order is completely destroyed by temperature fluctuations, and a transition of the substance to the paramagnetic state is observed:
- magnetic “gas” of the crystal;
- magnetic “liquid” of the crystal.
Temperature changes primarily affect the magnetization of ferromagnets. As it increases, the magnetization property decreases and becomes equal to zero at the Curie point. In this temperature regime, there is a change in all other properties that determine the difference between ferromagnets and paramagnets, as well as the characteristics of the substance that are not related to the distinctive features of these types of magnets. For example, a change in the electrical and acoustic properties of a ferromagnetic material, due to the fact that a solid body has elastic, electrical, magnetic and other subsystems, when one of them changes, the others also change.
Curie temperature
Each ferromagnet has a number of characteristics. An important parameter of a substance is the temperature at which it loses its magnetic properties. This indicator is called the Curie point. At temperatures above the Curie point, the ordered state in the magnetic subsystem of the crystal is destroyed.
Using metal as an example
The loss of ferromagnetic properties depending on the ambient temperature can be examined experimentally. For example, nickel has a Curie temperature of 360 degrees. A suspended metal sample is exposed to an external magnetic field. A burner is placed in the system. At normal temperatures, nickel will take a horizontal position, as it will be strongly attracted by a magnet. If a sample is heated to the Curie temperature, its magnetization property weakens, it stops attracting and begins to fall. After cooling to a temperature below the Curie point, nickel again acquires ferromagnetic properties and is attracted to the magnet.
Search magnet for gold and silver and its properties
Typically, powerful magnets are designed to find precious metals. A search magnet reacts to gold and silver quite strongly, and although it is difficult to find them in their pure form, its power is enough to pick up jewelry and coins from the ground. The main goal of all search engines is treasures, expensive coins, and sometimes just ferrous metal.
The article will describe the structure of the magnet and the basic principle of operation. He will also figure out what exactly can be found with its help and how to find expensive alloys. It will be explained in detail what ferromagnets, paramagnets and diamagnetic materials are. In addition, valuable tips and recommendations will be given that will greatly simplify the search for valuable items.
Does a magnet attract gold and silver?
Is it possible to find pure gold or silver with powerful magnets? No, since such metals are diamagnetic, that is, they are not attracted to magnets. But it's not all bad, thanks to all the power of neodymium alloy, it is possible to get some jewelry. Such objects usually have a ligature in them.
This alloy helps precious metals such as gold or silver acquire certain properties. For example, silver jewelry does not darken as much, but gold jewelry is more durable. But the most important thing is that the ligature allows magnetization and makes it possible to find various alloys.
But it is also possible to find pure gold or silver. At the beginning of the article it was said that iron boxes can be found. Typically, jewelry made of gold or silver is stored in such cases. So, walking through an attic or similar places, you can get rich, in the literal sense of the word.
Magnetic properties of various metals
In order to go hunting for valuable metals, you need to know what exactly will be attracted to a magnet. Since metals have different magnetic properties, and some do not have them at all. They can be divided into three groups:
Ferromagnets are metals with some of the best magnetic properties. Such metals are highly magnetic. These include ferrous metal.
Paramagnetic materials have the usual properties; they are readily attracted to a magnet, but do not have the function of magnetization. These include some alloys of jewelry and several types of non-ferrous metals.
And finally, diamagnetic materials. Such alloys are extremely difficult to respond to magnetic fields and greatly complicate the search for truly precious things. Diamagnets include gold, silver, aluminum, patina and other metals that even the strongest magnet does not pick up.
Is it possible to find gold with a magnet?
As already discussed earlier, jewelry and coins with gold can be lifted, but it is very problematic.
It is impossible to get pure gold with a magnet.
But if various factors are favorable, such as an iron box or paramagnetic jewelry lying nearby, then there is a chance to find it. Basically, only jewelry containing gold, such as bracelets, earrings and rings, can be caught with a magnet. The best places to search are sandy beaches, wells, and the sea or river bottom where a large number of people swim.
Chemistry notes in 11th grade: Metals – TeacherPRO
Key words of the abstract: General physical properties of metals. Classification of metals in technology. General chemical properties of metals. Conditions for the interaction of metals with solutions of acids and salts. Metallothermy
Metals, like all chemical elements, have three forms of existence: atoms, simple and complex substances. Of the 118 elements of the periodic table, 96 are classified as metals.
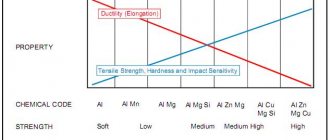
The physical properties of metals are determined by the metal crystal lattice and the metal chemical bond. Let us recall that metals are characterized by metallic luster, plasticity, high electrical and thermal conductivity, an increase in electrical resistance with increasing temperature, and in addition, such practically significant properties as malleability, hardness, and magnetic properties.
Metals are solid substances under normal conditions (except for mercury, which becomes hard and malleable at low temperatures).
Metals are ductile and malleable, except for the brittle bismuth and manganese. The thinnest sheets - foil - are made from copper, aluminum, tin, and gold. Gold foil can be around 100nm thick! This foil is used for gilding interior items, walls and ceilings, products made of plaster, wood, metal, glass and plastic.
All metals have a metallic luster, most of them are silvery white or gray in color.
Due to the fact that strontium, gold and copper absorb short wavelengths close to violet to a greater extent and reflect long wavelengths of the light spectrum, these metals are colored light yellow and copper.
Very thin sheets of silver and gold have a completely unusual appearance - they look like bluish-green foil, and fine metal powders appear dark gray and even black. And only magnesium and aluminum powders retain their silvery-white color.
In technology, metals are usually classified according to various physical properties:
- a) density - light (p 5.0 g/cm3);
- b) melting point - low-melting (mp 1000 °C).
Metals are usually divided into ferrous (iron and its alloys) and non-ferrous (other metals and alloys). The branches of the metallurgical industry are also named accordingly: ferrous and non-ferrous metallurgy .
The most important products of non-ferrous metallurgy are titanium, tungsten, molybdenum and other metals, which can be used as special alloying additives for the production of super-hard, refractory, corrosion-resistant alloys, widely used in mechanical engineering, machine tool building, and the defense and space industry.
Modern composite materials made on the basis of ceramics or polymers become super-strong if they are reinforced with metal threads made of molybdenum, tungsten, titanium, special steels, etc.
In all reactions, simple substances - metals - exhibit only reducing properties.
- Metals react with nonmetals to form binary compounds. According to IUPAC rules, the names of these compounds are formed in accordance with the scheme:
Thus, with very active non-metals (halogens, sulfur), metals form compounds that can be considered as salts of oxygen-free acids: 2Na + Cl2 = 2NaCl
If a metal exhibits variable oxidation states, such a salt has a composition that depends on the oxidizing properties of the non-metal. For example, iron reacts vigorously with chlorine, forming iron(III) chloride: 2Fe + 3Сl2 = 2FeCl3
When iron reacts with sulfur, whose oxidizing ability is lower than that of halogens, the reaction product is iron(II) sulfide: Fe + S = FeS
- When metals interact with oxygen, oxides or peroxides are formed :
4Li + O2 = 2Li2O 2Na + O2 = Na2O2
The oxides in this case have a basic or amphoteric character: 2Mg + O2 = 2MgO 4Al + 3O2 = 2Al2O3
These reactions are accompanied by the release of a large amount of heat and a very bright flame, therefore they are used for the manufacture of signal flares, fireworks, salutes and other pyrotechnics. Therefore, handling them requires strict adherence to safety regulations.
The product of combustion of iron in oxygen is a mixed oxide: 3Fe + 2O2 = Fe3O4
- Metals are simple substances formed by elements of groups IA and IIA; in full accordance with the names of these groups, they interact with water to form alkali and hydrogen . In general, these reactions can be written as follows:
2M + 2H2O = 2MOH + H2↑ , where M is an alkali metal
M + 2H2O = M(OH)2 + H2↑ , where M is Mg or alkaline earth metal.
To characterize the chemical properties of metals, their position in the electrochemical voltage series is important:
K, Ca, Na, Mg, Al, Zn, Fe, Sn, Pb, (H2), Cu, Hg, Ag, Au
Remember the two conclusions you know from the basic school course:
- the interaction of metals with acid solutions occurs if the metal is in the voltage series to the left of hydrogen;
- The interaction of metals with salt solutions occurs if the metal is in the stress series to the left of the salt metal.
Laboratory method for producing hydrogen: Zn + 2HCl = ZnCl2 + H2↑ Zn0 + 2H+ = Zn2+ + H20
The reaction of metals with organic acids proceeds similarly: 2CH3COOH + Zn -> (CH3COO)2Zn + H2↑ 2CH3COOH + Zn -> 2CH3COO– + Zn2+ + H20
The reaction between zinc and a solution of copper(II) sulfate proceeds according to the equation: Zn + CuSO4 = ZnSO4 + Cu Zn0 + Cu2+ = Zn2+ + Cu0
We emphasize that in this case the metal can be in the voltage series after hydrogen, but not after the metal salt. For example, the reaction of replacing silver with copper: Cu + 2AgNO3 = Сu(NO3)2 + 2Ag Cu0 + 2Ag+ = Cu2+ + 2Ag0
In conclusion, we will consider another property that is not characteristic of all metals, which is called metallothermy . Active metals such as aluminum, calcium, magnesium, lithium are capable of interacting with oxides of other metals.
In order for such a reaction to begin, a mixture of active metal and metal oxide (called thermite) must be ignited. After this, the process is accompanied by the release of a large amount of heat and light (hence the name of the process).
Metallothermy is used to obtain more valuable metals: 2Al + Cr2O3 = Al2O3 + 2Cr
Summary of a chemistry lesson in grade 11 “Metals”. For educational purposes, quotes from the textbook “Chemistry” were used. 11th grade: educational, for general education. organizations: basic level / O. S. Gabrielyan, I. G. Ostroumov, S. A. Sladkov. — M.: Enlightenment.” Select next action: