Nickel is a silver-gray, ductile, malleable metal. It belongs to transition metals, that is, it can exhibit both acidic and alkaline properties. Under normal conditions, nickel is covered with an oxide film and is therefore inactive. The difference from other similar elements is that its oxide film does not reduce shine. And today we will tell you about the use of nickel in industry, the use of its alloys in construction and other areas of life.
A little history
Many years before this miraculous discovery, miners from Saxony were familiar with ore that could be mistaken for copper ore. Attempts to extract copper from this material were futile. Feeling deceived, the ore began to be called “kupfernickel” (in Russian - “copper devil”).
Mineral expert Krondstedt became interested in this ore. After much work, a new metal was obtained, which was called nickel. Bergman took over the research baton. He further purified the metal and concluded that the element resembled iron.
Physical properties of nickel
Nickel is part of the tenth group of elements and is in the fourth period of the periodic table under atomic number 28. If you enter the symbol Ni in the table, this is nickel. It has a yellow tint with a silver base. Even in air, the metal does not become faded. Hard and quite viscous. It lends itself well to forging, making it possible to produce very thin products. Perfectly polished. Nickel can be attracted using a magnet. Even at a temperature of 340 degrees with a minus sign, the magnetic properties of nickel are visible. Nickel is a metal that is resistant to corrosion. It exhibits weak chemical activity. What can you say about the chemical properties of nickel?
Like all metals
Nickel is a metal. Therefore, it has general metallic properties:
- Ductility;
- Metallic shine;
- Plastic;
- Thermal conductivity (small);
- Electrical conductivity;
- Elasticity.
Physical properties
As a simple substance, the metal has a shiny light silver tint. It is resistant to alkaline, water, acid corrosion. Possessing plasticity, malleability and its own properties:
- Melting point 1450 °C.
- Boiling point 2830 °C.
- Thermal conductivity 92 W/m.
- Density 8.9 kg/dm3.
Optical properties of metal
To determine the atoms of an element, the disodium salt of acetic acid, anthranilic acid, is used. Optical density is measured at X=1000 mmk. The element is found in the presence of calcium, chromium, mercury, aluminum, and zirconium. But it is impossible to find with copper, iron (see Iron ores - the basis of modern production), cobalt, bismuth, silver. In reflected light it has a bluish color.
Crystallization studies
Crystalline bodies are metals and alloys. Their atoms are located at the vertices of crystal lattices. During the crystallization process, different crystal lattices are formed. It has a face-centered cubic shape. It forms two allotropic modifications:
- a-Ni at temperatures below 245°C;
- p-Ni.
A study of the crystallization process of liquid nickel was carried out. The number of atoms in a liquid increases as the temperature decreases. It is concluded that at first the element has a loose structure. Then the groups of atoms are restored.
Chemical properties
What is needed to determine the qualitative composition of nickel? Here we should list which atoms (namely their number) our metal consists of. The molar mass (also called atomic mass) is 58.6934 (g/mol). We have moved forward with measurements. The radius of the atom of our metal is 124 pm. When measuring the radius of the ion, the result showed (+2e) 69 pm, and the number 115 pm is the covalent radius. According to the scale of the famous crystallographer and great chemist Pauling, the electronegativity is 1.91, and the electronic potential is 0.25 V.
The effects of air and water on nickel are practically negligible. The same can be said about alkali. Why does this metal react this way? NiO is created on its surface. This is a coating in the form of a film that prevents oxidation. If nickel is heated to a very high temperature, then it begins to react with oxygen, and also reacts with halogens, and with all of them.
If nickel gets into nitric acid, the reaction will not take long to occur. It is also readily activated in solutions containing ammonia.
But not all acid affects nickel. Acids such as hydrochloric and sulfuric acid dissolve it very slowly but surely. And attempts to do the same with nickel in phosphoric acid were not successful at all.
Symptoms and signs of acute and chronic nickel poisoning
The simplest and relatively harmless type of poisoning is an allergy to nickel. Dermatologists know that this metal is one of the most common causes of allergic contact dermatitis. Even 2008 passed under the auspices of nickel, which was recognized as “allergen of the year” by the American Society for the Study of Contact Dermatitis, which indicates the importance of the problem. It is precisely due to the allergic properties of this metal that in the European Union countries the maximum permissible concentrations of nickel in those metal products that are in direct contact with human skin are legally limited. These are various bracelets, keychains, keys, door handles, rivets and zippers, eyeglass frames and other products. Dermatitis can be primary and secondary (systemic).
Nickel in nature
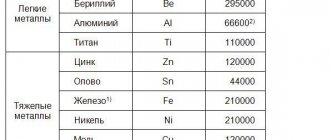
Scientists' speculations are that the core of our planet is an alloy that contains 90% iron and 10 times less nickel. There is the presence of cobalt - 0.6%. During the rotation process, nickel atoms were released into the earth's covering layer. They are the founders of sulfide copper-nickel ores, along with copper and sulfur. Some bolder nickel atoms did not stop there and made their way further. The atoms strove to the surface in company with chromium, magnesium, and iron. Next, the companions of our metal oxidized and became disconnected.
On the surface of the globe there are acidic and ultrabasic rocks. According to scientists, the nickel content in acidic rocks is much lower than in ultrabasic rocks. Therefore, the soil and vegetation there are quite well enriched with nickel. But the journey of the hero under discussion in the biosphere and water turned out to be not so noticeable.
Copper for anniversary
Since ancient times, it has been customary to give copper products to a spouse on their wedding anniversary. Today, the traditions have not lost their relevance. This is due not only to the belief that copper strengthens the bonds of marriage, but also has a beneficial effect on the body, or rather:
- has positive properties on the psyche;
- helps improve metabolism.
This is why modern alternative medicine recommends wearing copper jewelry. Copper is most often given as a gift for the seventh wedding anniversary, however, you don’t have to wait that long, you can please your other half with something made of bronze. The alloy has not lost its positive properties, therefore it will also provide the above properties. In addition, bronze accessories or souvenirs have an attractive appearance and are guaranteed to please your wife.
Additionally, it can be noted that some couples order the casting of unique coins, on which the number of their first kiss, wedding or acquaintance is engraved. Such gifts are believed to bring wealth to the family.
But if we put aside superstitions, the coin will not lose its relevance as a gift due to its originality.
Nickel ores
Industrial nickel ores are divided into two types.
- Sulfide copper-nickel. Minerals: magnesium, pyrrhotite, cubanite, milerite, petlandite, sperrylite - this is what is contained in these ores. Thanks to the magma that formed them. Sulfide ores can also yield palladium, gold, and more.
- Silicate nickel ores. They are loose, clay-like. Ores of this type are ferruginous, siliceous, and magnesian.
Where is nickel used?
Nickel is widely used in such a powerful industry as metallurgy. Namely, in the manufacture of a wide variety of alloys. The alloy mainly contains iron, nickel and cobalt. There are many alloys based on nickel. Our metal is combined into an alloy, for example, with titanium, chromium, molybdenum. Nickel is also used to protect products that corrode quickly. These products are nickel-plated, that is, they create a special nickel coating that prevents corrosion from doing its nasty work.
Nickel is a very good catalyst. Therefore, it is actively used in the chemical industry. These are instruments, chemical utensils, devices for various applications. For chemicals, food, delivery of alkalis, storage of essential oils, tanks and reservoirs made of nickel materials are used. Nuclear technology, television, and a variety of devices, the list of which is very long, cannot be used without this metal.
If you look into such a field as instrument making, and then into the field of mechanical engineering, you will notice that anodes and cathodes are nickel sheets. And this is not the entire list of uses for such a simply wonderful metal. The importance of nickel in medicine should not be underestimated.
Forged products - a gift for centuries
This section should be given special attention due to the fact that forged products can be given to absolutely everyone, thanks to the variety of designs that are manufactured today. Thus, cast iron items can be great for your girlfriend, grandparents, friends and even your boss. For example, for a private home it would be important to order an original lantern along with a bench. Spending warm summer evenings on such a colorful product will be delightful; the originality of the idea is guaranteed to please the heroes of the occasion.
You can also give wonderful steel boxes where your wife or mother can store precious jewelry. Just look at the work of a master of his craft. What is the advantage of such gifts?
Let's take a quick look at why you should pay attention to such designs.
- Cast iron forged products have a long service life due to their resistance to mechanical stress. Such a thing can be inherited by grandchildren or even great-grandchildren without losing its original form.
- The practicality of gifts has no limits. You can forge various objects: barbecues, benches, lanterns, figurines, decorations for the lawn in the backyard, artificial flowers as a gift for a girl and much more; in this case, you can do everything that the craftsman’s imagination is enough for.
- Iron structures will look great in any interior , be it an old wooden house or a cottage created in a modern way, in the courtyard of a private plot or on the balcony of a multi-storey building.
As you can see, gifts of this type are suitable for everyone. They will emphasize the individuality of the overall interior or simply delight the eye of their owner, and most importantly, they will bring a smile to the hero of the occasion.
Nickel in medicine
Nickel is used very widely in medicine. First, let's take the tools needed to carry out the operation. The result of the operation depends not only on the doctor himself, but also on the quality of the instrument he uses. Instruments undergo numerous sterilizations, and if they are made of an alloy that does not include nickel, then corrosion will not take long to occur. And tools made from steel, which contains nickel, last much longer.
If we talk about implants, nickel alloys are used for their manufacture. Nickel-containing steel has a high degree of strength. Devices for fixing bones, prostheses, screws - everything is made of this steel. In dentistry, implants have also taken a strong position. Clasps and stainless steel braces are used by orthodontists.
Conditions and restrictions for scrap acceptance
The procedure for accepting scrap metal has been approved by the Government of the Russian Federation and applies to enterprises engaged in:
- processing;
- resale;
- collection of scrap.
Acceptance of nickel scrap is carried out both from legal entities and from citizens who have reached legal age, on the basis of a written application
.
An individual is also required to present an identification document.
Representative of the organization
must also have with you:
- accompanying documents for scrap;
- power of attorney for him;
- power of attorney to receive money;
- a copy of the act on the write-off of equipment that is handed over as scrap.
The delivery of scrap metal follows the following rules:
:
- Purity
. Nickel and its alloys must not be contaminated with foreign elements and alloys, or contain dangerous, toxic impurities. The metal must be free of traces of paint, oil and flammable materials. - Before delivery, scrap must be sorted
by type, group, brand, category, grade. Lump metal is more expensive, shavings and wire are cheaper. - Quality
. The higher the nickel content in the alloy, the higher its cost. - size
. For large quantities of scrap metal, many scrap companies are willing to pay more, adding an additional percentage to their standard purchase price. They will offer the maximum price for a ton of non-ferrous metal.
It is better to deliver scrap nickel and its alloys to official companies
, which:
- have everything necessary to carry out such activities;
- have the necessary technological and material capabilities.
Recycling plants offer higher prices for scrap metal, while intermediaries buy at lower prices.
Nickel in living organisms
If you look at the world from the bottom up, the picture emerges something like this. There is soil under our feet. The nickel content in it is higher than in vegetation. But if we consider this vegetation under the prism that interests us, then a large nickel content is found in legumes. And in cereal crops the percentage of nickel increases.
Let us briefly consider the average nickel content in plants, marine and terrestrial animals. And of course, in a person. The measurement is in weight percent. So, the mass of nickel in plants is 5*10-5. Land animals 1*10-6, sea animals 1.6*10-4. And in humans the nickel content is 1-2*10-6.
Mined everywhere
The element Ni is not found in pure form, only in oxides, sulfides and silicates. Nickel mining occurs on all continents, and smelters are located in 25 countries. Large volumes of secondary and primary metal enter the world nickel market. Nickel is stored in known deposits. Where is nickel mined? Main nickel mining areas:
- Canada,
- Russia,
- Australia,
- Cuba,
- Albania,
- Greece,
- Ukraine.
Nickel deposits on the seabed exceed the content of the metal on land. The nickel reserve base could support the Earth for more than a hundred years. So nickel consumption may be high.
Unique deposits influence the development of the mining industry. Nickel mining in the world is carried out in 410 deposits. Unique deposits in Russia are located in Norilsk-1, Talnakh and Oktyabrsky. There are 2 deposits in the world in Canada, 3 in Australia, 1 in China, Greece, New Caledonia, 2 in Indonesia.
Where is nickel mined in Russia? Currently, the country has rich reserves of the resource, ranking first in the world in its production. Nickel mining in Russia occurs in the Taimyr district (69%), Murmansk region (17%), and the Urals (11%).
Nickel production in Russia is carried out:
- OJSC MMC Norilsk Nickel (95%);
- OJSC Yuzhural-Nickel;
- OJSC "Ufaleysky Nickel Plant";
- CJSC PA "Rezhnikel"
Nickel production in the world:
- Vale Inco Ltd in Canada;
- Western Mining Corp. in Australia;
- Jinchuan Group Co. Ltd" in China;
- "Goro Vale" in New Caledonia.
The role of nickel in the human body
I always want to be a healthy and beautiful person. Nickel is one of the important trace elements in the human body. Nickel usually accumulates in the lungs, kidneys and liver. Accumulations of nickel in humans are found in the hair, thyroid and pancreas. And that's not all. What does metal do in the body? Here we can safely say that he is a Swede, a reaper, and a trumpet player. Namely:
- tries, not without success, to help provide cells with oxygen;
- redox work in tissues also falls on the shoulders of nickel;
- does not hesitate to participate in regulating the body’s hormonal levels;
- safely oxidizes vitamin C;
- one can note its involvement in fat metabolism;
- Nickel has an excellent effect on hematopoiesis.
I would like to note the enormous importance of nickel in the cell. This microelement protects the cell membrane and nucleic acids, namely their structure.
Although the list of worthy works of nickel can be continued. From the above, we note that the body needs nickel. This trace element enters our body through food. Usually there is enough nickel in the body, because you need very little of it. Alarm bells of a lack of our metal are the appearance of dermatitis. This is the importance of nickel in the human body.
General information
Nickel is a ductile and malleable metal with a silvery-white color. Chemical activity is low: it reacts slowly with acids, and does not react with alkalis. When exposed to air, the element becomes covered with an oxide film.
The origin of the name of the compound is associated with an evil spirit - a gnome, who in German mythology seemed to throw to Saxon miners looking for copper a similar mineral - red nickel pyrite NiAs, the so-called arsenic-nickel luster. As a result of unsuccessful attempts to smelt copper from this ore, furious miners assigned the names “Kupfernickel” and “Nickel” to the new metal, which meant “Copper Devil” and “Mischief”, respectively. Today the word “Nikkel”, in the language of German miners, still means a curse word.
In human organs, this microelement is concentrated in the greatest quantities in the pituitary gland (substantia nigra of the midbrain), liver, pancreas, and adrenal glands. Nickel that enters the body with food is absorbed in the human digestive tract by 1–10%. At the same time, orange juice, milk, coffee, tea, and ascorbic acid reduce its absorption. Pregnancy, breastfeeding, iron deficiency, on the contrary, increase the absorption of the mineral.
Nickel is transported directly with serum albumin. It is interesting that in blood plasma the element is contained predominantly in a bound state with the proteins alpha-1-glycoprotein and nickeloplasmin (alpha-2-macroglobulin).
The “waste” compound is 95% excreted from the human body with feces, and the remaining 5% with bile, sweat, and urine.
Despite the positive properties of the trace element, remember that nickel is an active allergen that causes eczema and contact dermatitis in people sensitive to this metal. Possible reasons for the development of adverse reactions are contact of household objects, rivets on clothing, jewelry containing the element with the skin.
Nickel alloys
There are many different nickel alloys. Let us note the main three groups.
The first group includes alloys of nickel and copper. They are called nickel-copper alloys. Whatever the ratios in which these two elements are fused, the result is amazing and, most importantly, without surprises. Homogeneous alloy is guaranteed. If there is more copper than nickel in it, then the properties of copper are more pronounced, and if nickel predominates, the alloy exhibits the character of nickel.
Nickel-copper alloys are popular in the production of coins and machine parts. The alloy Konstantin, which contains almost 60% copper and the rest nickel, is used to create equipment of higher precision.
Consider an alloy with nickel and chromium. Nichromes. Resistant to corrosion, acids, heat resistant. Such alloys are used for jet engines and nuclear reactors, but only if they contain up to 80% nickel.
Let's move on to the third group of alloys. These are alloys with iron. They are divided into 4 types.
- Heat resistant – resistant to high temperatures. This alloy contains almost 50% nickel. Here the combination can be with molybdenum, titanium, aluminum.
- Magnetic - increase magnetic permeability, often used in electrical engineering.
- Anti-corrosion - this alloy cannot be avoided in the production of chemical equipment, as well as when working in an aggressive environment. The alloy contains molybdenum.
- An alloy that retains its dimensions and elasticity. Thermocouple in the furnace. This is where such an alloy comes in. When heated, the dimensions are maintained and elasticity is not lost. How much nickel is needed for the alloy to have such properties? The alloy should contain approximately 40% metal.
Gifts from spouses
Exchanging symbolic gifts on every wedding anniversary is a sweet tradition that helps strengthen the family.
A husband can give jewelry to his beloved wife. If desired, you can find stylish earrings or a bracelet made of nickel. But first you need to make sure that your wife is not allergic to close contact with nickel. If you are not so sure, then it is better to purchase jewelry made of silver or white gold. And in order not to break traditions, you can buy a cute trinket made of nickel, for example, a figurine or a candlestick. A beautiful bouquet of flowers or a box with your wife’s favorite sweets will complement the gift.
You can also give jewelry to your husband on his nickel wedding day. It could be a ring or a bracelet made of white metal. A great gift option is nickel cufflinks.
If your spouse does not wear jewelry, you can purchase other metal accessories depending on his preferences. Here are some gift ideas:
- high-quality lighter and ashtray made of white metal;
- metal flask;
- cigarette case;
- box for storing business cards;
- various office accessories - mobile phones, pen stands, etc.
It will be even more pleasant for the spouse if the gift has a dedicatory engraving.
By the way, gifts to your beloved spouse do not have to be material. Many will prefer a gift-impression to another souvenir. If possible, it would be nice to go on a short trip together. Cases are not allowed to leave the city? You can limit yourself to a romantic walk on a day off. For example, visit places where future spouses made dates for each other while they were not yet married, and then have a romantic dinner in a restaurant.
A great gift option is tickets to a concert of your favorite performer or an interesting performance. You just need to choose a program that will be interesting to both spouses. So, if your husband hates ballet, you should not buy him tickets to a three-hour performance as a gift. And the husband needs to think carefully about whether his wife will be happy to attend a football match if she is not at all interested in sports. However, people who have lived together for 12 years, as a rule, are well aware of the interests of their “half,” so the likelihood of a serious mistake in choosing an entertainment program is small.
Nickel in everyday life
If you look around, you can understand that nickel alloys surround people everywhere. Let's start with the furniture. The alloy protects the furniture base from damage and harmful influences. Let's pay attention to the fittings. Be it for a window or a piece of furniture. It can be used for a long time and looks very nice. Let's continue our excursion to the bathroom. There is no way without nickel here. Shower heads, faucets, mixers - all nickel plated. Thanks to this, you can forget what corrosion is. And there is no shame in looking at the product because it looks cute and supports the decor. Nickel-plated parts are found in decorative structures.
Nickel cannot be called a minor metal. Various minerals and ores boast the presence of nickel. I am glad that such an element is present on our planet and even in the human body. Here he plays an important role in hematopoietic processes and even in DNA. Extensively used in technology. Nickel gained its dominance due to its chemical resistance in protecting coatings.
Nickel is a metal that has a great future. After all, in some areas it is indispensable.
Use of material in construction
World nickel production in 1887 was only 600 tons. The metal was used to make coins. But already from the 80s, the nickel industry began to actively develop. The impetus was the high corrosion resistance of the metal, and, most importantly, its alloys.
- Nickel plating as a way to “ennoble” a product also began to be used from the end of the 19th century and was only replaced by chrome plating in the 30s of the 20th century. In construction, nickel-plated parts are still used in the construction of a wide variety of decorative structures.
- For the same reasons, nickel-plated parts are used in furniture production. The metal layer not only gives the product shine and beautiful color, but also protects the frame from any external influences.
- Decorative qualities determine another area of application - fittings for furniture, windows, doors, household appliances, and so on. Handles, hinges, and metal trims look great and last a very long time.
- Nickel-plated taps, faucets, shower heads and other bathroom accessories never go out of style, as the nickel layer provides the products with an excellent appearance and exceptional resistance to corrosion of any kind. Of course, this option is inferior to bronze in terms of decorativeness, because the base here is steel, and it is not malleable. But the silver color and unfading shine are also attractive.
- Alloys with nickel are used much more widely, especially various stainless and structural steels. It is unrealistic to imagine modern construction without the participation of rolled metal.
Nickel is a metal characterized by high corrosion resistance and is capable of imparting this property to its alloys. This quality is most often the reason for using metal.
This video will tell you about chemical nickel plating: