Technologies for preparing metal for coating
To ensure strong and uniform adhesion of the coating to the surface, the metal must be properly cleaned and prepared. The main types of contaminants that need to be removed from the surface of the part:
- mineral oils (rust protection products, coolants, lubricants, etc.);
- organic pollutants (paints, vegetable and animal fats, fingerprints);
- polishing compounds;
- solid particles (dust, shavings);
- oxide film, scale and rust.
To clean metals before coating, various methods are used, which are sometimes combined.
Mechanical cleaning
In this case, the removal of solid particles, burrs, scale and oxides from the surface of the part is carried out by processing with abrasive materials, sandblasting or vibration. Ultrasonic cleaning stands out on this list. In this case, the surface of the part is exposed to many small vacuum cavities formed as a result of the propagation of high-frequency (20–45 kHz) sound waves in the liquid in which the part is immersed. An aqueous solution of alkaline detergents at room temperature is used as a cleaning liquid. Ultrasonic cleaning is very effective at removing particulates, dirt and grime. For the processing of soft metals, cleaning is often used with aqueous solutions of alkaline or weak acids, which are applied to the surface of the part through high-pressure spray nozzles.
Polymer coatings
2.1. Coating materials
Polymer coatings are widely used to protect metals from corrosion. They not only protect the metal from corrosion and other chemical influences, but also give the product excellent electrical insulation, decorative, antiseptic and other properties. Compared to paint and enamel, polymer coatings have a number of advantages. They are more durable, elastic, better bonded to metal; During operation, they wear out and crack much less than enamels.
Metal plastics consist of a base, a metal substrate and a polymer coating. The base materials are most often steel or aluminum sheets, which give the metal-plastic the necessary strength and rigidity. The advantage of steel sheets is their high strength and low coefficient of thermal expansion compared to aluminum. Compared to steel sheets, aluminum sheets have low weight and a smoother surface, higher resistance in acidic environments and industrial atmospheres.
As a metal substrate in metal sheets, a zinc coating is used, obtained by immersion in molten metal or by electrogalvanization. The thickness of electrolytic zinc rarely exceeds 8-10 microns. Galvanization is especially important at the ends and near holes where polymer coatings may be absent.
Extremely high demands are placed on polymer coatings. The plastic must be resistant to abrasion, shock loads, the action of chemical reagents, temperature and climatic operating conditions. The last requirement is very important, since all polymer materials are more or less susceptible to atmospheric aging. With aging, the dielectric properties of the polymer significantly deteriorate, the natural color changes and the resistance to various chemical reagents decreases.
In addition to the type of polymer used in the manufacture of metal plastic, the choice of the type (state) of the material made for application to the metal is very important. Four types are used: plastisol, organosol, ready-made film, powder.
Plastisol is a solvent-free or nearly solvent-free resin dispersed in substances called plasticizers. Plastisol has a paste-like appearance and is used for applying thick (over 80 microns) coatings. The presence of plasticizers makes the surface of the plastisol coating relatively soft.
Organosol contains less plasticizers and more solvent. The thickness of the organosol coating is 30-50 microns. The coating surface in this case is harder than when applying plastisol.
Film coating has limited use. It is used for products that are used primarily indoors in order to improve their decorative properties. The film thickness is 50-500 microns.
The powdered substances used for coatings are classified as solvent-free coating systems. The advantage of this type of coating is the full use of the material and the ability to increase the thickness of the coating to 150 microns in the absence of porous areas and cracks.
The most widely used coating for metals is polyvinyl chloride (PVC). He has a number of valuable qualities. Polyvinyl chloride coating allows you to obtain a wide range of thicknesses and colors. It is resistant to acids, alkalis, solvents, has good dielectric properties, fairly high strength and elasticity. The disadvantage of PVC is its low heat resistance. The maximum temperature for long-term operation is +80 ºС, short-term +100 ºС. There are three main types of vinyl coatings used: organosols, plastosols and films.
The use of organosols and plastosols is due to their high physical and mechanical properties, weather resistance, chemical resistance and good anti-corrosion protection. The great advantage of these materials is their high dry matter content (in plastics 95-100%, in organosols up to 60%), which allows, when applying one or two layers of material, to obtain coatings with a thickness of 60-300 microns or more (plastisols) and 50-80 ( organosols).
Film PVC is a plasticized polyvinyl chloride resin with the addition of stabilizers, fillers and pigments. Plasticizer molecules, distributed between polymer molecules, reduce the adhesion forces in the film (hence the film strength decreases) and give the polymer molecules greater freedom of movement. This increases the elasticity of the film.
In addition to PVC films, polyethylene films are used as coatings. They have high resistance to most aggressive media and their vapors, solvents, oils, etc. Polyethylene
slightly adsorbs moisture, water vapor almost does not penetrate into it (about 10 times less than into polyvinyl chloride films). Dielectric properties are also much superior to those of polyvinyl chloride. Polyethylene film practically does not burn, and at high temperatures it only melts.
Chemical cleaning
Chemical cleaning is used to remove organic contaminants including traces of mineral oils, fingerprints, etc. using solvents. Most often, aliphatic solvents are used to prepare metal for coating. This group includes substances such as gasoline, acetone, and white alcohol. The use of certain solvents, such as trichlorethylene and trichloroethane (chlorinated hydrocarbons), is prohibited or restricted in most countries around the world due to their adverse effects on human health. There are various methods of applying solvent to the metal surface. So, some parts are completely immersed in the liquid, while the composition is sprayed onto others. Some solvents can also be used in gaseous form. This is called steam degreasing. There are other types of chemical cleaning. Thus, with emulsion technology, contaminants are removed by organic solvents dispersed in an aqueous solution containing emulsifiers (surfactants that prevent the dissolved component from sticking together). The workpieces are then subjected to an alkaline cleaning procedure to remove organic components of the emulsion from the surface before coating. Application is carried out by immersion or spraying followed by rinsing with water. The operating temperature of alkaline solutions is 50–84 °C.
High temperature coatings
1.1. High temperature coating materials
The operational reliability of machines is limited by the service life of parts operating under extreme conditions, i.e. at elevated temperatures, pressures and speeds.
Such parts are nozzle blades of gas turbine engines, elements of the piston group of internal combustion engines, etc. The service life of such parts can be significantly increased by applying heat-resistant coatings that protect the surface from high-temperature oxidation, erosion, and softening of the base material. The materials for high-temperature coatings are oxides and solid compounds: carbides, borides, nitrides, silicides.
Refractory oxides . Alpha aluminum oxide (α-Al2O3) is characterized by high mechanical strength, has several modifications, and has a melting point of 2050 °C.
In nature, aluminum oxide occurs in the form of corundum, sapphire or ruby.
Beryllium oxide (BeO) is a stable, slightly volatile compound with a melting point of 2570 °C. Its melting point is higher than that of aluminum oxide, and its mechanical strength is slightly lower than that of aluminum oxide. Due to its high electrical resistance, low electrical losses and low density, it is a better insulator than aluminum oxide, especially at high temperatures (1700-2000 ° C).
Cerium oxide exists in two forms: Ce2O2 and CeO2. The latter is more stable and melts at a temperature of 2593-2737 °C. Although cerium oxide is considered a rare earth material, it is practically more common in the earth's crust than zinc or tin.
Hafnium oxide HfO2 is commonly present in zirconium-containing minerals (2-7%); As a rule, hafnium oxide is not separated from zirconium oxide. Since the melting point of HfO2 is 2810 °C and it forms a solid solution with zirconium oxide (ZrO2), its presence does not deteriorate the properties of ZrO2. Pure hafnium oxide is a rare and expensive material.
Nickel oxide (NiO) is an easily reduced oxide. Its melting point is 1949 °C. Nickel oxide is the only refractory material that can be reduced to a metallic state in a hydrogen atmosphere. The volumetric changes accompanying this reaction are so great that they cause destruction of NiO products.
Thorium oxide (ThO2) is the most refractory, characterized by a melting point of 3200 °C. The limitations of its use are determined by its high density, high cost, and weak resistance to thermal shock.
Titanium oxide (TiO2) is a chemically stable crystalline substance that melts at 1840 °C. It is valued as a dielectric.
Solid compounds - carbides, nitrides, borides and silicides have similar structures, since the nitrogen and carbon atoms are in interstitial positions in the metal lattice. These materials have low electrical resistance; Moreover, most carbides, borides, and nitrides are distinguished by high thermal conductivity and great hardness.
Carbides are the most refractory substances: 4 TaС* ZrC (tmelt=3918 °C), 4 TaС*HfC (tmelt=3928 °С). Silicon carbide (SiC) and boron carbide (BC) are known as abrasives, and titanium carbide (TiC) and tungsten (WC) are known as components of hard alloys.
Nitrides are similar in properties to carbides, but their tendency to oxidize is greater than that of carbides. The most stable nitrides are HfN and ZrN. These materials can be successfully used for work in vacuum up to a temperature of 1800°C. When in contact with carbon, nitrides interact with it, forming carbides; Usually this reaction occurs even in a nitrogen atmosphere.
Borides are stable in the temperature range 2000-3200 °C. As a rule, all borides are characterized by low volatility, low electrical resistance, high hardness and good thermal conductivity. Borides are one of the few materials that are sufficiently stable and slightly volatile at 2500°C, and therefore can be effective heat-resistant materials.
Boron silicides B6Si and B4Si are suitable for use at high temperatures and are stable for a long time, at 1400 °C. They have high resistance to thermal shock. When oxidized, they form a protective body of borosilicate glass.
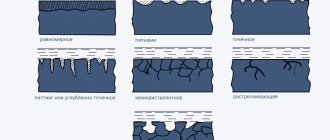
The developed heat-resistant and heat-protective coatings are divided into three groups (Fig. 1):
- single-layer metal type CrAlYZr HfSi (Fig. 1 a);
- two-layer coatings with internal metal CrAlYZrHfSi and external ceramic ZrO2 - Y2O3 (Fig. 1 b);
- three-layer coatings with internal metal CrAlYZrHfS, intermediate metal CrAlY; external ceramic ZrO2 - Y2O3 (Fig. 1c).
a B C
Figure 1 - Schemes of heat-resistant and heat-protective coatings: a - single-layer, b - two-layer, c - three-layer
The total thickness of single-layer coatings does not exceed 150 microns, two-layer - 200 microns and three-layer - 300 microns. The thickness of the inner layer in three-layer coatings ranges from 30 to 50 microns, intermediate 50-80 microns, external 80-120 microns. The concentration of chromium, aluminum, sodium, zirconium, hafnium, silicon in the heat-resistant layer is respectively 18-24% wt, 10-130% wt, 0.4-1.8 wt, zirconium, hafnium, silicon from 0.05 to 0. 2% wt.
Additional alloying of CrAlY alloys with zirconium, hafnium, and silicon made it possible: on the one hand, to increase the heat resistance of single-layer multicomponent and composite heat-resistant coatings, and on the other hand, to slow down diffusion processes at the boundaries of the base - damping inner layer - outer ceramic layer - and thereby increase the service life of the coating generally.
An even more significant slowdown of diffusion processes in the layers that make up the coating is observed when the intermediate heat-resistant layer is made in the microlayer version from 0.5 to 1 μm. In this case, optimal characteristics are achieved at a concentration of dispersed refractory particles (ZrO2 - Y2O3, Al2O3) in the microlayer from 0.3 to 1% wt.
1.2. Technological basis of thermal spraying processes
Depending on the heat source, the following methods of gas-thermal coating are distinguished:
- flame spraying,
- plasma,
- electric arc metallization,
- detonation-gas,
- vacuum condensation spraying.
The essence of gas-thermal coating processes is the formation of a directed flow of dispersed particles of the sprayed material and their transfer to the surface of the workpiece at temperatures and speeds that are optimal for the formation of a coating layer.
A general diagram of the thermal spraying process is presented in Figure 2.
Figure 2 - Scheme of the thermal spraying process: 1 - nozzle part of the particle generator; 2 - three-phase jet (Ι - condensed particles, ΙΙ - atoms of atomizing gas, ΙΙΙ - vapor phase); 3 - coating; 4—spray surface; Φ is the divergence angle of the particle flow; dпн — diameter of the spray spot
The sprayed material in the form of a powder, wire or rod will enter the heating zone and disperse. A distinction is made between radial and axial material feed. The heated particles are sprayed with gas. The main purpose of the gas is to accelerate particles in the axial direction. Atomizing gas, when using wires or rods, can disperse the molten material.
The coating is formed from powder particles that are in a molten state with partial melting, unmelted and partially solidified. When spraying wires and rods, the coating is formed from molten particles with a wide range of dispersion.
A number of requirements are imposed on the flow of sprayed particles to ensure favorable conditions for coating formation:
- the heating temperature of the particles must be sufficient to form strong adhesive bonds with the spraying surface. At low particle velocities (50-500 m/s), the heating temperature should be higher than the melting point of the material. High particle velocities (800-1000 m/s) make it possible to form coatings at temperatures up to 0.9 the melting point of the particle material;
- the speed of particles in the flow is determined depending on their temperature. It should be 50-100 m/s (minimum for molten superheated particles, maximum for unmelted particles in a viscoplastic state);
- The flow divergence angle φ should be 5-20 º. The minimum divergence angle ensures compact particle flows and the possibility of forming coatings.
The most important parameters of the sprayed material: physical and chemical properties, size of powder particles, diameter of the rod and wire.
Use wire with a diameter of 0.5-5 mm. The wire feed speed is selected as maximum for the given operating mode (2-10 mm/s).
The parameters characterizing the external conditions of spraying are: ambient pressure; spraying distance; spraying angle; temperature of the product, its shape and size; speed of movement of the spray spot.
1.3. Gas flame spraying
A gas flame is produced by the combustion of combustible gases in oxygen or air. The following flammable gases are used for spraying coatings: acetylene (C3H3), methane (CH4), propane (C3H8), butane (C4H10), hydrogen (H2), etc. The physicochemical properties of combustible gases are presented in Table 1.
Table 1 - Physico-chemical properties of flammable gases
| Flammable gas | Relative density in air | Calorific value at 25 ºС and 0.1 MPa, MJ/m³ | Heat of release during neutral combustion, MJ/m³ | Flame propagation speed in a mixture with oxygen, m/s | Temperature of gas flame mixed with oxygen, °K |
| Acetylene | 0,91 | 56,5 | 18,5 | 13,5 | 3100-3200 |
| Methane | 0,56 | 35 | 1,4 | 3,3 | 2000-2100 |
| Propane | 1,57 | 93,5 | 12,6 | 3,7 | 2400-2700 |
| Butane | 2,1 | 125,1 | – | – | 2400-2700 |
| Hydrogen | 0,07 | 10,8 | – | 8,9 | 2000-2100 |
Powder, flexible cords in a polymer sheath filled with powders, as well as flux-cored wires in a metal sheath are used as the sprayed material in flame spraying. The sprayed material is fed along the axis of the gas-flame jet into the torch (Fig. 3).
Figure 3 - Schematic diagram of gas-flame spraying: 1 - nozzle, 2 - gas torch; 3 - coating; 4 - substrate
The technological parameters that have the greatest impact on the efficiency of the process include: the diameter of the gas nozzle, the diameter of the holes on the periphery of the nozzle, the angle of inclination of the axis of the holes to the axis of the atomizer.
The transport gas pressure is selected within the range of 0.1-0.2 MPa, gas flow 0.3-0.6 m³/h, supply speed 180-500 m/h, mass of consumable material 5-30 kg/h.
The spraying distance is 100-200 mm, the speed of movement of the spray spot is 0.2-0.3 m/s. With powder spraying, the temperature of the sprayed material does not exceed 2200 ºС, with wire spraying - 2700 ºС.
Sprayers are being created that provide acceleration of gas-flame flows to supersonic and even hypersonic speeds. Fine powders are used as the material. This can provide coatings with low porosity (2-3%), with small pores (less than 10 microns), and roughness Rz 25...30.
The disadvantages of the flame spraying method include: the presence in the jet of active gases that interact with the sprayed materials; low values of effective heating efficiency of powder particles (1..15%); low quality coatings made from powder materials.
1.4. Electric arc metallization
The process is carried out by melting the coating material with an electric arc while simultaneously spraying it with gas. The most widely used two-electrode scheme (Fig. 4).
Figure 4 - Scheme of the two-electrode electric arc metallization process: 1 - electric arc; 2 — electrodes; 3 - nozzle; 4 — feed mechanism; 5 - contact devices; 6 - sprayed mixture
The sprayed material in the form of electrode wire 2 with a diameter of 1.0-5.0 mm is supplied by the feed mechanism 4 to the arc combustion zone 1. Voltage from the power source is supplied to the contact devices 5. Between the crossing electrodes there is a nozzle 3, designed to create a high-speed atomizing gas flow . For these purposes, compressed gas is used: nitrogen, argon, etc. The gas jet tears off the molten metal from the ends of the wires, disperses it and forms a stream of deposited particles together with the atomizing gas.
Along with two-electrode ones, three-electrode metallization schemes are also used. The electrode wires are placed along the generatrices of a truncated cone at an angle of 120º and use alternating three-phase current.
The shape and size of the nozzle have the greatest influence on the spraying process. Typically, cylindrical nozzles with a diameter of 3-6 mm are used.
Electric arc metallization is widely used in the formation of corrosion-resistant coatings of various building structures. A coating of aluminum and zinc is formed. Steel, bronze, etc. are used as wear-resistant coatings.
The advantages of the method are: high energy efficiency atomization, reaching 70-90%; high productivity up to 50 kg/h; the possibility of obtaining high-quality coatings with high adhesive strength and low porosity.
The disadvantages of the method are: interaction of particles with the active gas phase and saturation of the sprayed metal with oxygen and nitrogen; limited application of the method only to electrically conductive materials.
1.5. Vacuum condensation spraying of coatings
The coating during vacuum condensation deposition is formed by a flow of particles that are in an atomic, molecular or ionized state. The flow of particles is obtained by spraying the material in various ways: thermal evaporation of the material from a solid or molten state; explosive evaporation and melting; ion sputtering of solid material.
The process of vacuum condensation spraying is usually considered to consist of three stages: the transition of the condensed phase (solid or liquid) to gaseous (steam); flow formation and particle transfer to the condensation surface; vapor condensation on the spray surface and coating formation.
The process is carried out in rigid sealed chambers at a pressure of 13.3-13.3·10-3 Pa (Fig. 5). The highest vapor pressure, reaching 13.3 Pa or more, is observed near the evaporation surface. This causes the particles to move towards the surface of the product, where the vapor pressure is minimal.
Figure 5 - Scheme of the vacuum condensation deposition process: 1 - screen; 2 — working gas supercharger; 3 - coating; 4 - sprayed product; 5 - damper; 6 — flow of sprayed particles; 7 — supply of atomization energy; 8 — sprayed material; 9 - vacuum chamber; 10 - base plate
The introduction of active gases into the chamber makes it possible to switch to the method of vacuum reaction coating. Particles in the flow or on the condensation surface enter into chemical interaction with active gases (oxygen, nitrogen, etc.) and form the corresponding compounds: oxides, nitrides, etc.
Thermal evaporation of a material occurs when it is heated above its melting point. For most materials these temperatures exceed 1000-2000 ºС. The material is heated in an evaporator, the purpose of which is to hold the molten material until a vacuum of 1-100 Pa is achieved and ensure minimal heat losses. For example, the use of ceramic crucibles reduces the power of the heat source compared to water-cooled copper crucibles by 4-6 times. At the same time, copper crucibles allow materials to be evaporated without changing their composition. The use of several crucibles simultaneously makes it possible to obtain coatings with complex compositions and high uniformity in thickness.
1.6. Detonation gas coatings
Detonation-gas coating involves the use of specific sources of heating, atomization and acceleration of sprayed particles.
Figure 6 - Scheme of the spraying process: 1 - ignition chamber, 2 - igniter; 3 - shock waves; 4 - detonation wave; 5 - camera; 6 - flammable mixture; 7 — muzzle flame; 8 - flow of sprayed particles
A specified amount of a gas mixture, for example, C2H2 + O2 + N2, is supplied to the ignition chamber 1. Using a powerful electric discharge, the mixture is ignited through igniter 2. The flame spreads through the volume of gases with increasing speed. Shock wave 3 appears. Combustion turns into detonation. From this moment, a detonation wave 4 propagates along the barrel of chamber 5, representing a complex of a shock wave and a chemical reaction.
In the shock wave, the gas is compressed to a pressure of several tens of atmospheres, and the temperature rises to several thousand degrees and becomes significantly higher than the critical temperature at which the mixture reacts.
After the detonation wave reaches the open end of the barrel, the detonation wave is destroyed and a muzzle flame 7 is formed. The detonation products begin to flow out of the barrel in the form of a supersonic jet. The temperature and pressure of detonation products in the barrel decreases.
When expiring, the detonation products entrain the sprayed particles. A two-phase flow is formed, consisting of detonation products and sprayed particles. The two-phase flow is heterogeneous both along the length and cross-section of the barrel. Detonation products heat and accelerate the sprayed particles. After the two-phase flow leaves the barrel, the products of detonation coatings expand sharply. Their temperature, speed and density decrease with distance from the trunk. Near the surface of the workpiece, the gas flow slows down and then spreads along the obstacle.
When spraying materials of a homogeneous chemical composition, the coating can be formed from almost completely melted particles and from a mixture of molten and unmelted materials.
The cycle time consists of three components: the time required to fill the barrel chamber with the gas mixture; the time of explosion and release of detonation products and powders and the time of purging the chamber and barrel. Typically, the time of one cycle is 0.2-0.5 s. During one cycle, 30-40 mg of sprayed material is transferred to the surface. With one cycle, a single spot with a diameter of 20-30 mm and a thickness of 10-30 microns is formed.
There are single-barrel and multi-barrel installations, the latter providing increased productivity. The barrel diameter (8-40 mm) and its length 1200-2000 mm have a great influence on the efficiency of the process.
The advantages of detonation gas spraying are:
- high quality coating;
- increase in fatigue strength due to cold hardening;
- moderate heating of products during their spraying (t<300ºC);
- fairly high productivity (1-10 kg/h);
- wide range of sprayed materials;
- low sensitivity to the condition of the initial spraying surface (heating, contamination, roughness);
- unlimited size and shape of products. For example, both large products (up to 10 m long, 2-3 m in diameter, weighing up to 4 tons) and small ones (surgical instruments, drills, taps, etc.) are subjected to spraying.
The disadvantages should be noted:
- difficulty in applying coatings to products with a large surface and high hardness (HRC>60);
- difficulty in spraying coatings from low-density powders (titanium carbides, etc.);
- impossibility of spraying internal surfaces to a depth exceeding the diameter of the inlet;
- high noise level (140 dB and above);
- the need to use sealed boxes and remote control of the process;
- quite high cost of equipment.
1.6.1. Application of detonation coatings
The scope of application of detonation coatings is very wide. The range of parts with detonation coatings is constantly expanding. The detonation coating method, initially tested and successfully mastered in the aviation and space industries, has found wide application in other industries.
A significant argument in favor of the use of detonation coatings is the possibility of obtaining structures with low weight combined with high wear resistance. The effect is enhanced by the fact that the technological process of detonation coating practically does not change the original configuration of the part and does not have a noticeable effect on the microstructure of the material due to local thermal effects. The part itself rarely heats up to temperatures above 200ºС. If necessary, the temperature of the part is reduced by blowing it with carbon dioxide or air. Thanks to this, it is possible to coat parts manufactured with high precision and maintain it.
Some surfaces of parts made of low-melting materials (for example, the inner surface of an aluminum alloy bushing with a diameter of about 10 mm) can only be properly coated using the detonation method, since other gas-thermal methods require the parts to be located in an intense heating zone.
Detonation coatings are most widely used in the field of increasing the service life of turbine blades of aircraft engines, compressors, etc.
Detonation coatings facilitate the introduction of lighter alloys into aircraft engine structures and components. For example, a coating based on tungsten carbide has made it possible to successfully use titanium blades in turbojet engines. The use of levers made of aluminum alloys with wear surfaces coated with hard alloys made it possible to reduce the overall weight of the aircraft.
In mechanical engineering, the main task of using detonation coatings is to reduce the wear of rubbing mating parts for various purposes. Increasing speed, thermal and power loads require the materials of moving machine parts to combine very contradictory properties: low mass and high strength, heat resistance and heat resistance, wear resistance, low friction coefficient, etc.
Experience in using detonation coatings based on aluminum oxide has shown that they have high wear resistance over a wide range of loads and friction rates in combination with various materials. Comparative tests of sliding bearings, the working surface of which was covered with a wear-resistant coating and operating in a corrosive environment, showed a 30-fold increase in service life.
The use of detonation coatings for tools allows us to solve the problem of an optimal combination of rigidity and wear resistance. Possessing high hardness and adhesion strength to the base, applied coatings made of hard alloy and aluminum oxide will allow you to obtain high-quality edges on cutting tools for various purposes.
Thus, the service life of cutting blades used for cutting plastics, rubber, paper and coated with a layer of hard alloy based on tungsten carbide with a thickness of 0.05 mm increases from 3 to 12 times.
Detonation hard alloy coatings applied to the surface of carbon steel inserts used as saw teeth when cutting cardboard, asbestos fabric and similar materials increase service life by 50 times.
Detonation coatings on fiberglass cutting knives increase their service life by 10 to 20 times.
Ultrasonic-assisted tungsten carbide-coated tubular drill bits last 50 times longer than conventional tools.
When drawing wire through dies coated with chromium oxide, the resistance increased by 3-7 times.
Punches with detonation coatings have 10 to 20 times longer service life than chrome-plated steel ones.
Electrochemical cleaning
Electrocleaning is carried out in an alkaline electrolyte through which a direct current is passed. The part is connected to the anode or cathode. This method combines the effects of chemical and mechanical cleaning with gas bubbles that form on the surface of the part as a result of an electrochemical reaction. Electrochemical cleaning allows you to remove organic contaminants embedded in the surface of the part, solid particles adhering to the surface, and oxides. At the end, the workpiece is rinsed with water and washed with an acid solution. There are several types of electrocleaning.
Anodic (reverse)
In this method, the part is connected to the positively charged side of the power supply. The following reaction occurs: 4[OH] = 2H2O + O2 + 2e Oxygen bubbles form on the surface of the part, which contributes to the cleaning effect.
Cathode (direct)
In this case, it is connected to the negatively charged side of the power supply. The following reaction occurs on the anode surface: 4H2O + 4e = 4[OH] + 2H2 In the process, hydrogen bubbles are released in an amount twice the amount of oxygen in the first method. The cathodic one is more efficient than the anode one due to more intense gas release. The disadvantages of this method are the possible deposition of impurities on the metal surface and the fragility of the part caused by hydrogen diffusion.
Combined
In this method, the part is alternately connected to the anode and cathode at controlled intervals. The method combines the advantages of both anode and cathode technology.