Corrosion of a metal is the destruction of its structure as a result of chemical or electrochemical reactions. This can lead to the destruction of parts, structures, and lead to failure of automobile components, machine tools, other production equipment, tools, pipelines and other metal products. Every year, corrosion destroys about 13 million tons of metal.
Anti-corrosion protection measures for metal are designed to prevent and slow down this negative process. More than $2.5 trillion is spent on this worldwide each year, according to NACE International. As practice shows, metal products are simply not able to serve without special protective measures for a long time
Types of corrosion
The corrosion process has been ruining people's lives for many centuries, so it has been studied quite widely. There are different classifications of corrosion depending on the type of environment, on the conditions of use of the corrosive materials (whether they are energized, if they are in contact with another environment, then permanently or alternatingly, etc.) and on many other factors.
Electrochemical corrosion
Two different metals connected to each other can corrode if, for example, condensation from the air gets into their joint. Different metals have different redox potentials, and at the junction of the metals a galvanic cell is actually formed. In this case, the metal with a lower potential begins to dissolve, in this case, to corrode. This shows up on welds, around rivets and bolts.
To protect against this type of corrosion, galvanizing is used, for example. In a metal-zinc pair, zinc must corrode, but when zinc corrodes, an oxide film forms, which greatly slows down the corrosion process.
Chemical corrosion
If the surface of the metal comes into contact with a corrosive environment, and there are no electrochemical processes, then the so-called. chemical corrosion. For example, the formation of scale when metals interact with oxygen at high temperatures.
Anti-corrosion paints
In the modern world, metal processing with paints and varnishes can be carried out both on prepared and loose surfaces. Modern paints contain active substances and corrosion inhibitors that can create an ideal surface with maximum protection from further destruction. Enamel primers contain not only a protective function, but also decorative qualities. Such paints are applied to metal, giving it not only protection, but also, if it is the product of an enterprise, a marketable appearance.
Anti-corrosion
Despite the fact that ships with chests rotting at the bottom of the sea are not so bad for the environment, metal corrosion causes huge losses to people every year. It is therefore not surprising that various methods of protecting metals against corrosion have long been available.
There are three types of corrosion protection:
The construction method includes the use of metal alloys, rubber gaskets, etc.
Active methods of combating corrosion are aimed at changing the structure of the electrical double layer. A constant electric field is applied using a direct current source, the voltage is selected in order to increase the electrode potential of the protected metal. Another method is to use a sacrificial anode, a more active material that will be destroyed, protecting the product being protected.
Passive corrosion control is the use of enamels, varnishes, galvanizing, etc. Coating metals with enamels and varnishes is aimed at isolating metals from the environment: air, water, acids, etc. Galvanizing (like other types of spraying), in addition to physical isolation from the external environment, even if its layer is damaged, will not allow metal corrosion to develop, i.e. .To. zinc corrodes more readily than iron (see “electrochemical corrosion” above in the text).
Protective coatings can be applied to metal in various ways. Galvanization can be carried out in a hot shop, or in a cold shop, using thermal spraying. Painting with enamels can be done by spraying, roller or brush.
Much attention must be paid to preparing the surface for applying a protective coating. The success of the entire set of measures to protect against corrosion largely depends on how well the metal surface is cleaned.
Source: real-color.ru
Use of inhibitors at home
For general users, the most affordable means of corrosion protection using inhibitors is to apply a primer compound to the target surface. This is a lightweight inhibitory coating, the effect of which is to prevent direct contact of water or an aggressive solution with the metal surface. Often paints and varnishes contain similar corrosion inhibitors. Substances used for such purposes are produced in factories. These include red lead for the same primer, solutions of zinc or iron orthophosphates, phosphate coatings, etc.
see also
How to wash your car in winter
- 5 0 4k
Anti-gravel treatment
- 44 0 38k
How to wash poplar from a car
- 3 2 6k
The best wax for a car
- 41 0 43k
Which sound insulation for a car should you choose?
- 48 0 51k
The fight against car corrosion often causes a lot of problems for its owner. For this, three main methods are used - passive, active and electrochemical, but each of them has its own advantages and disadvantages. Most often, corrosion is removed using special means. And for preventive purposes, a protective film is glued to the bottom, body thresholds and other hidden places or treated with mastic. There are also other preventive measures, which we will talk to you about later.
Specialized drugs
If folk life hacks don’t help or the rust gets too deep, use a special anti-corrosion substance. You can purchase the product at any hardware store. During handling, be sure to wear protective gloves and a mask. Strictly follow the manufacturer's instructions so that you do not damage the metal while removing rust.
1. Neutralizers and converters
Chemicals should be used in complex and advanced cases
Chemicals that prevent the spread of corrosion. After applying the product to metal, the substance reacts with iron and forms a protective layer on the surface of the material. The film will remain on the product for a certain period of time, and then the procedure will have to be repeated. The products protect the material from the negative effects of oxygen and moisture.
2. Paint
Paint will prevent rust from spreading
You can use oil paint or a special rust stain. Regular paint should be applied to a surface that has been previously cleaned of corrosion. However, the disadvantage of this method is the possibility of rust progression under the painted layer. It is better to use special paints that can be applied directly to areas with corrosion. The product prevents the spread of rust by stopping the oxidation of the metal.
3. Primer
Primer enamel converts rust into a protective layer
In addition to regular primers, there are also varieties for working with rust. The operating principle of the product is to convert corrosion into a protective film. Prime the surface to level it, and treat it with special paint on top.
Causes of corrosion
First, let's figure out why corrosion processes occur. The fact is that there are four types of corrosion of metal surfaces - electrochemical, chemical, hydrogen and oxygen. In the context of rusting of an automobile body, only the first two types occur.
Electrochemical corrosion occurs due to the fact that two materials with different reducing properties interact through an electrolyte (any non-distilled water is such). Since iron has low reducing properties, it is significantly susceptible to rusting. Chemical corrosion occurs due to the interaction of the metal surface and a corrosive environment. The latter role can be played by oxygen at high temperatures. Understanding the essence of the emerging processes gives us the basis for searching for methods to combat corrosion.
Types of corrosion control
There are two main ways to protect a car body from corrosion. The first is barrier protection. It does not allow physical interaction of the surface of vulnerable metals with the external environment. This is expressed in the use of paint and varnish coating and various mechanical means and protection. The second is tread protection. An example of this is galvanizing, because zinc has a more negative potential than iron. Accordingly, if you connect them, then in such a pair the iron will be reduced and the zinc will corrode. However, since there is an oxide film on the surface of zinc, this process occurs very slowly.
As mentioned earlier, there are three main types of car corrosion control:
Corrosion Removal Brushes
- Passive.
- Active.
- Electrochemical.
The passive control method involves the use of paintwork on the body. The car owner’s task in this case is to maintain the integrity of the paintwork. Do not allow small chips or scratches to appear on its surface. This method also includes periodic washing of the car, as well as the use of additional protective agents - wax, liquid glass, and so on.
An active method of combating car corrosion means the use of special anti-corrosion materials and mastics. They differ depending on what areas of the body they are used for. For example, the underbody of a car is often treated with an anti-gravel coating. As a rule, these compositions are based on fine aluminum powder. There are also special anti-corrosion agents for wheel arches. Most often, the so-called liquid locker (strong elastic material) is used for this. A separate class are anti-corrosion materials for hidden cavities. They are designed for processing thresholds, pillars, side members, floor reinforcements and other surfaces.
Which rust converter is best to buy?
As you can see, all rust converters for cars cope with the task assigned to them to one degree or another. Some turn the damaged area into soil, others form a protective film and prevent the lesion from growing. However, the most important condition for their effective action is strict adherence to the rules for applying the drug and preparing the surface to be treated as specified by the manufacturer.
Depending on the quality of the effect and the method of application of the product, the following conclusions can be drawn:
- Many car owners consider the American-made Hi Gear aerosol to be the most effective rust converter.
- Among the domestic products, the highest quality and most effective drug is the rust converter Tsinkar.
- If you have to treat a small surface against corrosion, you should opt for Fenom FN956.
- Liquid rust converter “Khimik” is suitable for those who need a large amount of product to treat a surface significantly damaged by corrosion.
- The ability to remove rust in hard-to-reach places is guaranteed by Astrochem aerosol.
- For those who value not only quality, but also a convenient method of application, without bubbles or drips, the Permatex gel rust converter is suitable.
This rating examined the 6 best rust converters for cars, and all of them protect the vehicle from corrosion quite well. But here is the main advice that experienced mechanics give: when fighting iron oxidation, you should not delay treating the damaged surface for too long; it is better to neutralize small lesions than to restore large areas.
How to remove corrosion from a car
Now let's move directly to the methods and means of combating corrosion on a car with our own hands. First of all, it is necessary to mechanically remove rust from the surface. And do it very carefully! For these purposes, sandpaper, various abrasive wheels on a drill or grinder, as well as sandblasting are used. It is the latter tool that most effectively cleans the affected surface.
Special compounds are also used to remove corrosion. The simplest thing in this case is to use a weak solution of hydrochloric acid and then remove it.
However, the most reliable method of controlling corrosion is to use rust converters or rust modifiers. They convert iron oxide to iron tannate. As a rule, they contain polymers that act as a primer.
Car rust converters convert corrosion into a layer of iron and zinc phosphates and chromates. They are also sometimes used to treat uncorroded metal before applying primer to prevent future corrosion and improve the degree of adhesion of the paintwork to the metal surface.
Independent fight against car corrosion has the following sequence:
- Degreasing the surface. To do this, you can use various means, for example, alcohol or white spirit.
Removing rust from the body
Remember that all work must be carried out carefully, since even a small rust stain can grow significantly over time.
The rusty seam is visible
Always check the condition of the welds on the machine body. Remember that they are the most vulnerable to corrosion. In particular, its intercrystalline type, which is especially dangerous. The consequence of its appearance is an imperceptible loss of ductility and strength of the metal. Thus, the boundaries of welded grains are destroyed chaotically, and the areas of structural transformations turn into an anode, which intensively dissolves. Moreover, this phenomenon can be observed not only on iron car bodies, but also on stainless steel, aluminum, chromium-nickel and chromium alloys. Corrosion in this case threatens to chip away individual grains of metal, which is why the seam and the body as a whole gradually lose their mechanical properties.
The most rust-prone areas of the car body are the lower parts of the door panels, sills, front fenders, box sections of the lower body, and the inner surface of the wheel arches. Due to the fact that access to the listed places is difficult, there is always a risk of not noticing the appearance of rust spots. Check their condition in the inspection pit or on a lift!
Removing rust from metal surfaces
If we are talking about cars, then corrosion most often appears on the car body. It is clear that any car owner is interested in the “health” of his “pet”, and regular updates of anti-corrosion protection are entirely on the owner’s conscience. But it also happens that corrosion finds loopholes, or due to some circumstances, vulnerable areas appear on the protective layer.
If the slightest signs of damage are detected, it is necessary to take immediate action, otherwise the focus of active oxidation will not only expand, but also go deeper into the metal. If the metal sheet is eaten through by rust, then other, more expensive repair methods will have to be used.
Here you need to act quickly: if you come to your senses in time, you can still return the damaged area to its original appearance (provided you choose the right shade of paint).
Removing rust from a car body - applying converter with a brush
Any of the selected rust removers must be used in the prescribed sequence. Only in this case can you achieve the desired effect:
- The first step is to carefully clean the damaged area from loose rust using a wire brush and then sandpaper of the desired grain size.
- Next, the surface is treated with a rust converter;
- The next step is to wash the treated area several times (if specified in the instructions for the rust converter, since sometimes this is not required.)
- After this, the metal surface is dried with a rag or using a hair dryer;
- Once the surface is ready, they move on to painting work.
When cleaning, you may find that rust has already made a through hole in the metal. If it is very small, then you can try to seal it with putty using fiberglass. If the hole has reached a significant size, it is impossible to do without welding a patch, and this will require special equipment and, naturally, stable skills in carrying out such repair and restoration work. In order not to let things get to this point, you should carefully monitor the state of the car’s anti-corrosion protection in order to stop the onset of metal corrosion at an early stage.
Cleaning the area damaged by corrosion
Restoration of an area damaged by corrosion using a converter and soil is carried out in approximately the following sequence:
- Cleaning metal from loose layers of rust.
- Degreasing the cleaned area.
- Rust converter treatment.
- If necessary, the next step is puttying, and after it dries, sanding.
- Then the surface is degreased again.
- The next stage is the application of protective primer in one or two layers.
- Next comes two or three layers of adhesive primer;
- After this, the repaired area is painted in several layers.
- A special varnish is applied on top of the paint.
When performing all work using anti-corrosion chemicals, it is necessary to comply with safety regulations. A prerequisite is the use of protective equipment - rubber gloves, goggles and a respirator. If necessary, the metal surface surrounding the damaged area should be covered to eliminate the possibility of contact with the converter, primer, and finishing paints and varnishes.
* * * * * * *
It must be said right away that the complete restoration of a heavily damaged area with its subsequent finishing painting is a rather difficult task, and not everyone can do it. That is, if you have doubts about obtaining an acceptable result, it is better to turn to specialists. This is not cheap, and therefore the best solution is to try not to bring your equipment to a state that requires such intervention. That is, to nip the appearance and development of areas affected by corrosion, as they say, “in the bud.” There are a lot of resources for this.
Next, we will present a rating of the best compounds that can effectively combat the corrosion process that has arisen on the surface and in the hidden cavities of the car.
Popular rust removers
Currently, there are dozens of different rust converters in auto stores, and their range may vary in different regions of the country. Therefore, it makes no sense to give recommendations regarding the purchase of this or that product. But we will still give as an example several names of popular compounds that are common among car owners. So:
Popular remedy "Tsinkar"
- "Tsinkar";
- "Movil";
- Hi-Gear line of rust converters;
- "Chain mail";
- Sonax;
- "SF-1";
- Runway;
- Permatex;
- Bitumast;
- "Phosphomet".
It must be remembered that with the help of any converter you can fight rust, the layer of which does not exceed 0.1 mm. In addition, the active components only fight stubborn rust. It is better to remove its loose component mechanically (using sandpaper, a knife, a metal brush, sandblasting, and so on).
The choice of one product or another should be based on the range, its composition, and price. Fortunately, they are inexpensive, so if the purchased product turns out to be ineffective, you can always buy another one.
Source: etlib.ru
Corrosion control methods
CORROSION OF METALS AND ALLOYS
1. Corrosion
(from the Latin “corrodere” to corrode) is a spontaneous redox process of destruction of metals and alloys due to interaction with the environment.
2. Types of corrosion
: chemical and electrochemical
I.
_
Chemical – corrosion caused by the interaction of metals with substances contained in the environment, in which redox destruction of the metal occurs without the occurrence of electric current in the system.
Chemical corrosion includes:
– gas corrosion
– corrosion destruction under the influence of gases at high temperatures;
– corrosion in non-electrolyte liquids.
– chemical corrosion caused by the interaction of metals with gases.
Main oxidizing agent
- air oxygen.
Processes of chemical corrosion of iron:
2 Fe + O 2 = 2 FeO
3 Fe + 3 O 2 = FeO Fe 2 O 3 (mixed iron (II, III) oxide)
4 Fe + 3 O 2 + 6 H 2 O = 4 Fe (OH)3 (in air in the presence of moisture)
Fe(OH)3 t °C →
H 2 O + FeOOH (rust)
2 Fe + 3 Cl 2 = 2 FeCl 3
Chemical corrosion in non-electrolyte liquids
Non-electrolyte liquids
- These are liquid media that are not conductors of electricity. These include: organic (benzene, phenol, chloroform, alcohols, kerosene, oil, gasoline); inorganic origin (liquid bromine, molten sulfur, etc.). Pure nonelectrolytes do not react with metals, but with the addition of even a small amount of impurities, the interaction process accelerates sharply. For example, if the oil contains sulfur or sulfur-containing compounds (hydrogen sulfide, mercaptans), the process of chemical corrosion accelerates. If in addition the temperature increases, there will be dissolved oxygen in the liquid - chemical corrosion will intensify.
The presence of moisture in non-electrolyte liquids ensures intensive corrosion through the electrochemical mechanism.
Anti-corrosion
Corrosion is the process of destruction of a metal during its physical, chemical or chemical interaction with the environment. It is divided into three types: - chemical (without the occurrence of electric current); — electrochemical (accompanied by a corrosion current); — mechanochemical (corrosion-mechanical wear, added friction, cyclic bending loads, vibration, etc.).
The car is mainly characterized by electrochemical corrosion. It is especially intense at relative air humidity of more than 60% and in a polluted urban atmosphere. Corrosion is caused by:
— weather conditions (rain, snowfall, temperature changes);
- pollution from acids and alkalis contained in the air, or salt sprinkled on roads in winter;
— the appearance in the metal after stamping and welding of areas with a changed structure;
— surface heterogeneity, as well as micro-inclusions of slag and small defects (shells).
According to the nature of its spread, corrosion can be continuous or local:
— a continuous one appears on the entire body, starting from the lower surface of the bottom, from the inside of the wings, and in the internal cavities of the doors and power elements (thresholds, cross members, amplifiers). Inside the cabin, it usually occurs under the floor mats;
— local occurs in places where metal sheets are joined by welding and rolling (edges of the hood and trunk lid, door perimeter). It is more dangerous than solid, as it flows faster, leads to through damage to parts and, as a consequence, to a loss of strength and rigidity of the body.
Galvanized body parts, although more slowly, also rust, especially in industrial cities. In hidden cavities, corrosion is invisible and therefore most dangerous. When the car moves on uneven surfaces, micro-movements occur in the weld seams of the body elements, reducing the tightness of the parts and destroying the previously applied protective film. Once rust appears on external surfaces, the process is irreversible.
The underbody of the car corrodes when the factory plastisol coating ages, peels off, and when moisture gets into the formed cavities. In addition, the protective layer is damaged by sand, small pebbles and gravel flying from under the wheels; it is torn off by accidental contact with hard objects - for example, icy snow accumulations in uncleared yards, protruding roots and fallen branches on forest roads, in ruts or when parking on sidewalk curbs.
The external paintwork of the body suffers from the effects of salt, acid precipitation, dirt and dust, ultraviolet radiation, and temperature changes (daily and during washing). The paint fades, oxidizes, becomes covered with scratches and cracks. As a result, the car begins to rust not only in hidden cavities, but also on the outside.
When to treat
At car factories, bodies are primed and painted, mastic (plastisol coating) is usually applied to the bottom, and protective compounds are applied to hidden cavities. Some body parts are galvanized. Sometimes manufacturers guarantee the time until through-body damage occurs. Nevertheless, it is periodically necessary to do additional anti-corrosion treatment. It all depends on the car:
— new foreign cars rarely require additional anti-corrosion protection after purchase. During operation, individual weak points are identified - you can consult both the car dealership and the anti-corrosion center about the advisability of their treatment;
— it is better to process new domestic cars completely and immediately. Factory anti-noise plastisols covering the bottom and wheel arches do not penetrate welds, do not contain corrosion inhibitors and only protect the metal from mechanical stress. In addition, the composition is applied before painting, having previously covered numerous threaded holes and studs with technological stickers. When assembling the car, they are removed, simultaneously exposing sections of the bottom. When transporting painted bodies and installing them on a conveyor, local damage to the coating is also possible. If the hidden cavities of the body are processed at the factory, a passport with a protection scheme and warranty conditions for it is usually attached to the operating manual. In this case, it is useful to contact an anti-corrosion center and check the completeness of application and the condition of the coating;
- used cars. It is recommended to periodically (every year or two) carry out complete anti-corrosion treatment. However, some anti-corrosion centers retain their guarantee if the owner of the car changes. Therefore, if the previous owner handed over the relevant documents, you can rely on their recommendations.
The frequency and volume of treatment depend on the operating conditions of the vehicle, the completeness of the previous anti-corrosion protection, the preparations used and the warranty conditions of the company that performed the work. In any case, it is recommended once a year, preferably at the end of summer, to visit an anti-corrosion center for a routine inspection and repair of minor damage to protective coatings. In addition, in the spring it is useful to thoroughly wash the car to completely remove any remaining de-icing compounds. Otherwise, in summer, at elevated temperatures and periodic wetting (rain, dew), the corrosion process is activated. At the same time, it is possible to notice and eliminate any defects in anti-corrosion protection. When replacing or repairing body parts after an accident, it is also necessary to restore anti-corrosion protection.
What to process
Conventionally, three generations of compositions are distinguished.
First: conservation, made from thickened oils with additives of corrosion inhibitors. These materials do not last long on vertical surfaces (doors, thresholds). They flow down, leaving a film that is unstable to mechanical stress and permeable to water vapor.
Second: film-forming inhibited petroleum compounds (PINS), which adhere well to the metal being protected. The waxy film mechanically insulates it from the atmosphere, and inhibitors block corrosion. Sometimes rust modifiers are contained; they restore the metal, turning corrosion products into an additional protective film about 100 microns thick, similar to soil. Often, an aluminum filler is introduced into the base of the compositions (the word “bronze” or gold is added to the names), it increases abrasion resistance and makes it difficult for aggressive ions (for example, chlorine) to penetrate. Recently, preparations with zinc filler have appeared; its particles, increasing the abrasive resistance of the coating, help slow down electrochemical corrosion.
Third: materials that contain water or highly purified oils instead of volatile petroleum solvents. Such compositions do not poison the atmosphere. Large manufacturers of anticorrosion agents produce a full range of compounds that differ in the degree of protection. All modern preparations are compatible with factory coatings, and anticorrosives of the same brand are compatible with each other. But it is not recommended to change the brand of the composition unless absolutely necessary. Even specialists at anticorrosion centers cannot always determine how the car was protected. Therefore, it is often necessary to remove the old additional coating from the bottom. And it is almost impossible to remove it from hidden cavities. Sometimes you can find out what a car has been treated with by looking at the sticker on the rear window.
Materials for protecting hidden cavities (ML-preparations) must:
— penetrates well into microgaps and cracks;
- be homogeneous and preferably thixotropic (the ability to adhere to vertical surfaces and set quickly);
— displace water and electrolytes from the metal surface;
— effectively impregnate corrosion products (rust);
- form an elastic film;
— do not have a harmful effect on the paintwork;
- have reliable adhesion (adhesion to metal).
Materials for protecting the underbody and wheel arches (UB-preparations) must:
— have high adhesion of the protective film to metal and factory coatings;
- have mechanical strength and abrasive resistance to impacts of sand and gravel, do not crack or peel;
— be elastic and withstand operating temperatures and mechanical deformations of the body;
- It is good to isolate the metal from aggressive electrolytes.
Anti-gravel protection materials must protect the factory paintwork from the intense abrasive effects of sand and gravel. This is another processing step. Anti-gravel agents often contain polymer components to increase durability.
Paint protection materials penetrate into the pores of the paint and provide additional protection. They must be water-repellent, UV-resistant and contain corrosion inhibitors.
The main stages of complete anti-corrosion treatment:
— washing the car from below with hot (60–80°C) water under pressure up to 60 atm with the fender liners removed;
- drying.
However, modern anti-corrosion materials displace water, so they can be applied to a damp surface;
— inspection and troubleshooting (some anti-corrosion drugs glow blue under ultraviolet irradiation);
- application of drugs.
There are two ways to access the “labyrinths” for processing: with and without drilling additional holes. As a rule, the first method is used. The second is much less common, because almost all cars have inaccessible volumes that require special equipment to process; — after application, the preparations gradually (about a day) set. During this period, it is better to refrain from operating the car. When forced to travel through snow, water, dirt and gravel roads, you must move carefully. In addition, you can drive a car with a catalyst no earlier than three hours after treatment. The car cannot be washed for a week.
Where to do
As a rule, an anticorrosion center is chosen based on the recommendations of friends or based on one’s own experience. The last method is the most correct, because the quality of the work is confirmed by time. In addition, a good company has indirect distinctive features:
— positive reputation, work experience and official representative status (direct supplies of anti-corrosion compounds);
— the opportunity to familiarize yourself with the list of work performed and technological maps (schemes) for processing vehicles;
— certificates of specialist training and company certification;
— a complete set of equipment for all types of processing (main types of nozzles for spraying drugs);
— cleaning and washing the car immediately after treatment or, if provided for by the technology, after a certain period of time;
— competent staff answers to questions about the purpose of the materials used and their differences, the essence of processing, etc.;
— a guarantee for the safety of the body and its preventive maintenance, the conditions of which are detailed in the relevant document.
Do-it-yourself anti-corrosion treatment
Let's say right away that it is better not to do this: self-treatment is less effective than that done in an anti-corrosion center, where special equipment and well-established technology are used. But if you still decide to do the processing yourself, then it is useful to consider that:
— it is not recommended to reduce the amount of preparatory and main work by skipping individual stages;
— it is advisable to reproduce as much as possible the conditions and processing modes adopted in anticorrosive centers;
- the benefits of “folk” remedies (mining, gun fat, bitumen, shale mastics) are small - they do not contain inhibitors, create a greenhouse effect and can peel off the factory plastisol coating;
— modern preparations sold in aerosol packaging are intended only for minor repairs of the corresponding anti-corrosion coating. They have a low concentration of protective material - no more than 30%. The remaining volume is occupied by solvent and propellant;
— it is advisable to use formulations identical to those used in anti-corrosion centers, but packaged in small-volume containers (so-called Euro-cylinders with a capacity of 1 liter).
When purchasing drugs in a store, you need to pay attention to the accompanying information. It should contain:
— information about the manufacturer (company name, address, contact numbers, etc.);
— characteristics of the drug: name, purpose, color, consistency, type of solvent;
— standard or specification number, date of manufacture, shelf life, batch number;
— technological application features: type of surfaces to be treated and their preparation, effect on paint and varnish coatings, method of application and removal, temperature ranges of treatment, drying modes, thickness of dry and/or wet film, number of layers, drying and holding time before use, consumption;
— precautions for storage and use, urgent measures in emergency situations.
Corrosion of metals. Ways to combat corrosion
How to organize distance learning during quarantine?
The Infourok project helps
Description of the presentation by individual slides:
Definition: Corrosion (from the Latin corrodere - to corrode) is the spontaneous destruction of metals and alloys under the influence of the environment.
Corrosion causes serious environmental consequences. Leakage of gas, oil and other hazardous chemical products from pipelines destroyed by corrosion leads to environmental pollution.
Corrosion of metals and alloys (their oxidation) is caused by environmental components such as water, oxygen, carbon and sulfur oxides contained in the air, and aqueous solutions of salts.
These components directly oxidize metals - chemical corrosion occurs. Electrochemical corrosion (the most common form of corrosion) always requires the presence of an electrolyte (Condensation, rainwater, etc.), as, for example, when iron rusts in a humid atmosphere.
Most often, iron products are subject to corrosion. Metal is especially corrosive in humid air and water. The chemical equation for this process is: 4Fe + 3O2 + 6H2O → 4FeO(OH)•H2O
Chemically pure iron almost does not corrode, but industrial iron, which contains various impurities, for example in cast iron and steel, rusts. Consequently, one of the reasons for the occurrence of corrosion is the heterogeneity of the metal.
Ways to combat corrosion: 1. Applying protective coatings on the surface of the metal being protected from corrosion. For this purpose, oil paints, enamels, and varnishes are used. These non-metallic coatings are inexpensive but usually short-lived.
Methods of combating corrosion: The protected metal can be coated with a layer of another metal: gold, silver, chromium, nickel, tin, zinc, etc. One of the oldest methods is tinning - this is coating an iron sheet with a layer of tin. Such iron is called tinplate.
Inhibitor compositions
Most often, compositions based on sodium nitrite are used, which are added to sodium silicates and phosphates, brines, sodium bichromates, sulfoxides, amines, tannin, etc.
Moreover, when using one or another inhibitor, it is important to take into account that the defense reaction involves its consumption, therefore it is periodically necessary to introduce new portions of the active element into the aggressive environment. For example, a typical composition of a corrosion inhibitor based on sodium nitrite is added in a volume of up to 0.05%
Also, the active groups of compounds behave differently in certain environments. So, if the task is oxidation, then hydroquinone is taken as a basis, and to delay rust processes in relation to steel alloys, the use of technetium is recommended. Specialized compounds include inhibitors for protection in environments with chlorine and hydrogen. In this case, nitrogen trichloride is used, but in minimal doses. As a rule, a thousandth of the total amount of reagents is enough to stop a negative interaction.
Ways to combat corrosion
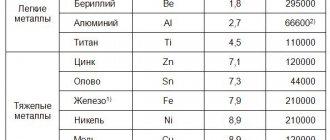
As an example, let's look at some of the features of corrosion of stainless steels and ways to combat it. The high corrosion resistance of stainless steels is determined by their ability to be easily passivated (covered with a protective film) even under normal atmospheric conditions due to oxygen in the air.
The corrosion resistance of stainless steels depends on:
• on the content of chromium, the main alloying component, with an increase in the content of which the corrosion resistance of steel sharply increases;
• carbon content, with an increase in which the corrosion resistance of steel decreases significantly;
• structural state of steels. Solid solutions alloyed with chromium and nickel have the greatest corrosion resistance. Violation of the homogeneity of the structure, due to the formation of carbides or nitrides, leads to a decrease in the chromium content in the solid solution and a decrease in corrosion resistance;
• the nature of the aggressive environment and the stability of the passive film. Stainless steels are stable in solutions of nitric acid, various neutral and weakly acidic solutions in the presence of oxygen, and unstable in hydrochloric, sulfuric and hydrofluoric acids. Steels lose their stability in highly oxidizing environments due to the destruction of passive films, for example in highly concentrated nitric acid at high temperatures;
• temperature, at an increase in which the corrosion resistance of stainless steels sharply deteriorates in both oxidizing and non-oxidizing environments.
Corrosion in stainless steels can occur through both electrochemical and chemical mechanisms.
Due to the complex structural state and the large difference in the electrochemical and corrosion properties of the structural components, stainless steels are especially prone to local damage (intergranular corrosion, pitting, pitting). In complex structures with gaps and cracks, crevice corrosion is typical.
Intergranular corrosion most often occurs in welded joints and in cases of improper heat treatment. In this case, the grains are in a passive state, and the grain boundaries are in an active state, due to the formation of chromium carbide. With increasing carbon content in steel, its sensitivity to intergranular corrosion increases sharply. The grain size has a significant influence on the sensitivity of steels to intergranular corrosion, and the smaller the grain size, the less sensitive the steel is to corrosion.
There are several effective ways to combat intergranular corrosion:
1. Reduced carbon content, resulting in reduced carbide formation at grain boundaries. Less sensitive – steels with carbon content
Direct and indirect problems associated with metal corrosion
The main problem with corrosion is the gradual destruction of corroded parts of structures and products. At the same time, the degree of damage cannot always be assessed by appearance, and the loss of strength becomes unexpected and critical.
Intercrystalline corrosion, that is, passing along the boundaries of crystals, is especially strong. Externally, the process may be completely unnoticeable, while the level of strength loss reaches 50...60%.
Surface destruction has the least impact on the strength properties of products.
The photo shows part of the structures of the Shukhov Tower in Moscow. Surface rusting has significantly reduced structural strength, but has not led to the destruction of the structure (yet)
It is extremely difficult to estimate the level of losses from metal corrosion. The point is not even in direct losses from the destruction of corroded parts or structures, but in downtime of equipment and structures and disruptions in their performance as a whole, associated with the corrosion destruction of individual elements.
DESIGN FUNDAMENTALS. CHEMICAL PRODUCTION. AND EQUIPMENT
Technological diagrams of processes for granulating dispersed materials
The main equipment for industrial compaction of dispersed materials includes a mixer, a compaction device (plate, press, extruder, etc.), conveyor, dryer or classifier. Dust collection systems are mandatory in installations, including...
Fluid bed granulation
In a fluidized bed, granules of fertilizers are obtained, such as carboammophosphate, urea, ammonium nitrate, nitrophoska, ammophos, as well as feed yeast, dosage forms, aluminosilicates, synthetic zeolite powders, etc. The essence of the process is ...