Corrosion characteristics
Therefore, it is necessary to immediately discuss what corrosion is? This process is a specific chemical reaction. It occurs only when favorable conditions appear for this process.
Corrosion can appear on various metal surfaces if:
- the material will interact with water over a certain period of time,
- when the metal surface is in an open area,
- If all operating conditions have not been met, the metal itself is deformed, and its properties change so much that it becomes unsuitable for further use.
The metal, undermined by the external environment, crumbles and changes color and texture.
Types of corrosion
There are two types of corrosion: chemical and electrochemical.
Chemical corrosion can occur in liquids and gases (steam). This process takes place in an environment where it is not possible to transmit electric current.
Gas corrosion occurs due to the action of gas (steam) on the metal surface, which causes an increase in temperature.
Liquid, in turn, arises due to the action of liquid on the surface of the metal, but does not transmit electric current. This type of corrosion is most often found in tanks for storing and transporting petroleum products and oil.
In two options, the process of material erosion will proceed at a rate proportional to the speed of the chemical reaction.
Protection methods
Let's look at the measures to protect this seemingly stable material. The external environment can destroy it gradually, thanks to corrosion and rust that occurs as a result of a chemical reaction on the metal surface.
To prevent the spread of corrosion over the entire surface of the material, anti-corrosion coatings are used. These special metal protection products help slow down and further prevent the spread of rust through the metal. Such corrosion control products are affordable in terms of price. They are easy to find in a regular hardware store. This product quickly reacts with rust, affecting its foci.
Not only specialized paints and varnishes can be used as anti-corrosion protection agents. The most acceptable and cheapest means are paints and enamels with special chemical additives.
Such anti-corrosion measures also have a number of advantages:
- They are very easy to apply and use, do not require long training before use or a large team of specialists;
- They allow you to quickly and efficiently process metal structures of large dimensions and complex designs;
In addition, one should not deny the fact that the use of such coatings provides the following bonuses:
- You can get a coating of any color - just choose from the palette and order;
- Such coatings are quite cheap,
- They have high protection characteristics,
- If they are damaged during operation, they can be easily restored.
It is also worth noting that these anti-corrosion protection procedures are used primarily as a means of long-term insulation of metal elements present in the structure. This method of metal processing can be advantageously combined with decorative coating finishes. The aesthetic beauty of the appearance plays an important role in the overall result of the finishing work performed. After all, if the repair is done carelessly, it is possible that corrosion will return again after a while and bring even more problems associated with subsequent cleaning of the metal surface.
Your car deserves to be taken care of. Even in the warm season, moisture and abrasive flying from under the wheels cause damage to the car, especially the bottom of the car, hidden cavities, places where two or more metals are connected, sills, edges of doors and hood, trunk. The moisture there almost never dries out, day after day imperceptibly devouring the metal, until one fine moment it crawls out. Can you imagine what’s happening there when a “cocktail of de-icing materials” or just salt and sand is poured onto the streets. A standard paint coating cannot withstand this, especially since an additional protective anti-corrosion coating in the form of varnish is not applied to the bottom and in hidden cavities at the factory. Only an additional anticorrosive agent can help here, which, due to its specially selected properties, will not only protect open surfaces but will also penetrate into all hard-to-reach places. As they say, some water will find a hole, but the anticorrosive will find this water, but unfortunately not any water at all and will not allow it to do its rusty work.
All cars need to be anticorrosive. New because this will allow you to keep them new as from the factory, and this is a huge plus for possible resale, and even more so if you drive it for a long time. If you don’t immediately do the anti-corrosion treatment, then at the next maintenance it will be very unpleasant to discover corrosion, even if it’s just superficial red rust on a car that’s shining with varnish on the outside. You can also treat rust against rust, but it is much more effective to do it preventively. And for cars that have traveled, you can extend the life of the body by stopping corrosion that has already begun and, accordingly, avoid expensive and difficult body repairs.
Our service center Antikorka.ru offers you professional anti-corrosion car treatment services at affordable prices. There is nothing supernatural in our work. You just need to approach the process carefully and honestly - don’t be lazy, remove the fender liners and trims, wash all surfaces and cavities, and thoroughly dry the entire body.
And then the main thing is not to skimp on materials. The prices are quite different, everyone, of course, wants to save money, but it’s better not to use very cheap materials to prevent car corrosion. A bad coating will dry out quickly and crack. Visually the car seems to be protected, but in fact it will rust.
Waiting for you! We will try to make anticorrosion efficiently and quickly!
Advantages of anti-corrosion coatings
The anti-corrosion coating has a whole range of positive properties.
Among them, the following stand out:
- resistance to water,
- resistance to various types of fuel,
- prevention of reactions with most chemical elements that can destroy the protective layer.
- electrical insulation,
- weather resistance.
Such materials are able to provide both passive and active protection against corrosion. As passive protection, a layer of paint and varnish physically isolates the metal from moisture. It is worth noting that the main types of paint protection used specifically for the passive protection of metal structures are materials using synthetic binders and alkyd-based paints. If you need a thin but high-quality coating, you should take a closer look at bitumen-based paints. If it is necessary to use it in an aggressive environment, at high temperatures, then you should pay attention to silicone enamels.
At the same time, active anti-corrosion protection itself implies the use of chemical inhibitors in paints that slow down the oxidation process of metals, as well as various other additives. It is worth noting that such coatings will last several times longer than any other layer of passive protection.
Types of anti-corrosion coatings
It is worth noting that there are different types of anti-corrosion coatings for metal. Anti-corrosion coatings to protect metal from the external environment are one of the most important areas of production activity in the paint and varnish industry.
Electrochemical corrosion
Corrosion processes are based on redox reactions of metals with the environment, accompanied by the transition of metals to a more thermodynamically stable state.
Let's consider iron corrosion as an electrochemical process. The rusting of iron is nothing more than an anodic reaction
Cathodic reaction – reduction of atmospheric oxygen:
Hydrogen ions are supplied by water. If there were no dissolved oxygen in the water, corrosion would be impossible. Consequently, iron corrodes in a layer of water saturated with oxygen. Thus, the initial stage of iron corrosion can be conveyed by the reaction
| 2Fe + O2 + 4H+ → 2FeO + 2H2O. |
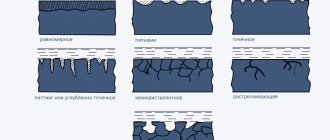
The corrosion rate is significantly affected by the concentration of H+ ions. Increasing the pH results in slower corrosion because the reduction of O2 from H2O is slowed down. At pH = 9–10, iron corrosion practically stops. It is known that in an aqueous environment, Fe2+ ions in the presence of oxygen are oxidized to Fe3+. The second stage of corrosion corresponds to the formation reaction of hydrated iron oxide (rust) Fe2O3∙nH2O (Fig. 7.4):
| 4Fe2+ + O2 + 4H2O + xH2O = 2Fe2O3∙xH2O + 8H+. |
Figure 7.4
Corrosion of iron by air oxygen dissolved in water
To protect iron from corrosion, all kinds of coatings are used: paint, a layer of metal (tin, zinc). At the same time, paint and tin protect against corrosion as long as the protective layer is intact. The appearance of cracks and scratches in it allows moisture and air to penetrate to the surface of the iron, and the corrosion process resumes, and in the case of a tin coating it even accelerates, since tin serves as a cathode in the electrochemical process (Fig. 7.5).
Figure 7.5
Corrosion of tinplate
Galvanized iron behaves differently. Since zinc acts as an anode, its protective function is preserved even if the zinc coating is damaged (Fig. 7.6).
Figure 7.6
Cathodic protection in galvanized iron
Cathodic protection is widely used to reduce corrosion of underground and subsea pipelines and steel supports of high-voltage transmission lines, oil platforms and jetties.
Figure 7.7
Cathodic protection of underground pipeline
Anti-corrosion metal coating
Often, in order to prevent the spread of corrosion through metal elements, it is necessary to comply with all operating rules that exist for a given device or part of a living space. Otherwise, no anti-corrosion paints will be able to hold back rust for a long time.
To begin with, you should ensure good ventilation in the room and at the same time take care of its tightness. This is especially true in the fall, when frequent rains and high humidity cause favorable conditions for corrosion.
Initially, a living space needs not only frequent cleaning and ventilation, but also full heating. Humidity should not be allowed to rise. Water is the first enemy of metal structures suffering from corrosion and rust, which provoked it.
It is important to remember that one coat of paint is insufficient protection of the metal from the harmful effects of the environment.
Therefore, it is better to spend more financial resources, but perform high-quality finishing work, which subsequently will not require repeated repairs, or a complete rework of the entire coating.
The thicker the protective layer, the better for the metal coating itself. However, too much paint should also not be applied. Three or four layers will be enough if the enterprise is industrial and the work here involves the development of chemical elements.
A few layers of paint can do more to protect a coating than hundreds of expensive paints with anti-corrosion additives.
How to defeat rust: the main methods of anti-corrosion protection of metal
Each product requires an individual approach, since the reason for the development of corrosion is different for each: it is determined by specific atmospheric and operational factors.
Our company’s specialists have extensive experience with anti-corrosion treatment of metal structures and help customers choose the required type and method of protection.
The main methods of anti-corrosion protection of metal structures can be identified:
- Electrochemical;
- Chemical;
- Reducing the aggressiveness of the environment;
- Application of coatings (metallic and non-metallic).
What should you pay attention to?
However, excessive use of existing production volumes will not only lead to overspending and a large increase in the time required to dry the metal structure, but also, too thick a layer of paint often causes cracks in the coating that form during drying.
Much depends on the quality of the paint applied. If before applying the coating to the surface you notice that it is too liquid and spreads quickly, the manufacturer may have added an excessive amount of water. Then you will have to purchase another paint or you should add special substances to your existing paint to prevent excessive spreading of the paint material.
At the same time, if you apply paint very thinly, this can lead to ineffective protection of metal structures, which affects the molecular bonds of the paint coating, and also leads to too rapid destruction - that is, the paint will simply rub off and the original appearance of the coating will be lost.
Protection of building materials from corrosion
This section describes methods for protecting building materials from corrosion in aggressive environments.
Any structure, except for purely forceful influences causing a volumetric stress state, is subject to physical and chemical influences of the environment.
The medium can be in gaseous, liquid or solid form, and most often - multiphase. So, for example, foundations can be affected by the soil adjacent to them (mainly groundwater that saturates it); Walls and coverings are exposed to external and internal atmospheres of varying humidity and pollution.
Individual environmental agents are characterized by greater or less aggressiveness towards various structural materials, i.e., the ability to cause their complete or partial destruction over a certain period of time.
Strictly speaking, every environment affects the structure. These effects can be either aggressive (most often) or favorable, helping to stabilize and even strengthen the material.
The main objectives were: assessment of the aggressiveness of different environments, description of the effect of the environment on materials and structures, drawing up recommendations for the selection of materials resistant to these environments; The main attention is paid to the effects of chemical agents and the protection of materials and structures from them.
The task of the designer who prescribes this or that material for use in structures with aggressive environments is responsible and quite complex. The use of relatively economical, but often insufficiently resistant materials leads to their rapid destruction and high costs for repair and restoration work; the use of highly resistant, but usually more expensive materials increases costs. construction of structures.
The ideal solution, apparently, would be to design equally resistant elements of structures that would collapse simultaneously and only after a long (specified) service life corresponding to the obsolescence of the equipment. However, in practice this is not always possible. Therefore, during the operation of buildings and structures, unfortunately, it is necessary quite often to replace quickly deteriorating elements or individual structures - usually floors, less often foundations, walls, coatings and others. In such cases, when designing major repairs at individual enterprises, it is desirable to provide for the possibility of section-by-section replacement of structures: this will speed up repair and construction work and will allow them to be carried out in the conditions of an existing enterprise without disrupting the overall flow of the technological process.
The medium acts primarily on the surface layers of structures, gradually penetrating into the depths, especially if the material is not dense enough. Therefore, very often, protecting structures from aggressive environmental influences comes down to compacting the material in the surface layer or applying protective coatings that are sufficiently dense and resistant to the given environment - linings, plasters, adhesive insulation or even painting. Naturally, during the operation of buildings and structures, these protective coatings must be periodically and timely updated.
Taking these circumstances into account, the mechanism of corrosion processes is discussed on the site in relation to individual materials from which structures are made - metal, concrete, wood - or to materials that are applied to structures as protective coatings - paints, plastics, mastics and solutions.
A significant place is occupied by a description of methods for increasing the density and durability of ordinary cement concrete, which is quite natural: it is used in construction; High-density cement concretes are quite economical and durable structural and protective (for metal) materials in many environments, with the exception of strongly acidic waters.
To increase the durability of materials and structures operating in acidic environments, special mastics, mortars and concretes based on liquid glass, sulfur cement and bitumen are recommended.
In the presence of variable (acid-base) influences or when exposed to highly concentrated or heated chemical solutions, it is necessary to use more resistant materials - based on plastics and plastic concrete.
Safety at work
In order to most effectively eliminate the likelihood of poisoning and illnesses arising from work related to the application of paint and varnish coatings, you will be required to strictly follow safety regulations.
First of all, in the rooms where work will take place, it is necessary to ensure good ventilation. Then, if this is a large room, then the people performing paint and varnish work must be provided with all the necessary personal protective equipment - that is, mittens and overalls.
Moreover, special attention from this list should be paid specifically to respiratory protection equipment - that is, masks and half masks - respirators. Also, you or your workers should remember about personal hygiene. To clean your hands from paint and varnish materials, you can use special cleaning pastes. But under no circumstances use solvents to clean the skin, as this leads to the appearance of a rash on the skin, as well as various allergic irritations.
In order to promptly identify various diseases of this nature, it is necessary that people who do paint and varnish work undergo periodic medical examinations in order to prevent the occurrence of these dermatological diseases.
Application of anti-corrosion coating
Metal protection requires special attention and a number of preventive measures to prevent corrosion. If this process could not be avoided, and rust has already appeared on the metal, you will have to work in several stages:
- Initially, flaw detection should be applied. This method is a detailed examination of a surface in order to detect the degree of corrosion present in the metal. At this stage, diagnostics are carried out and the most appropriate methods for further combating rust and its consequences are determined. In some cases, high-quality and complete finishing of a residential premises or an industrial department of an enterprise is required.
- Preparatory work related to preparing the surface for subsequent work aimed at cleaning the metal from rust and carrying out finishing work. Sometimes before painting you will need to prime or remove scratches through which moisture has penetrated into the structure of the metal itself, destroying it from the inside. Do not forget that dust is not so harmless and its removal is one of the main stages of preparatory work. After all, dust may contain various chemical elements and compounds that provoke the destruction of metal structures.
- The last and most important stage is the application of the appropriate paint and varnish substance to the surface of the material. There is no need to rush here; the subsequent effectiveness of the protective layer of paintwork depends on the quality of work. After each subsequent layer, you should wait until it dries and hardens and check the quality of the work performed.
Anti-corrosion coating thickness
Table. Features of coatings of various types of paints and varnishes.
| LMB | Advantages | Flaws |
| physical drying paintwork | ||
| Acrylic | Excellent weather and light resistance; Excellent decorative properties Good interlayer adhesion and adhesion to the painted surface; | Low dry residue (up to 50%); Small thickness of one layer (20-30 microns); Low resistance to solvents; |
| Copolymer vinyl chloride (CV, CS) | Possibility of application at negative temperatures (up to - 10°C); Quick drying; Good water and weather resistance; High elasticity and impact strength; Ease of repair; | The need for careful surface preparation; Small thickness of one layer (40-50 microns); Low resistance to solvents; |
| Chlorinated rubber | Possibility of application at subzero temperatures (down to -15°C); Good water, acid and alkali resistance; Reduced flammability of the coating due to the chlorine content; Relatively short interlayer drying time; Ease of repair; | Low resistance to solvents and petroleum products; Small thickness of one layer (50-70 microns); Low dry residue (no more than 50%); The need for careful surface preparation. Deterioration of physical and mechanical properties under the influence of sunlight; |
| Coatings cured with air oxygen | ||
| Alkyd | One-component; Relatively low cost; Tolerance to the quality of surface preparation (St 2): the oils included in the composition penetrate well into the rust, impregnate it and do not allow further spread; Good adhesion to metal, wood, mineral substrates; High technology, good spreadability; High decorative properties; Good interlayer adhesion; Ease of repair; | Long drying time; Application at temperatures above +5°C; High content of organic solvents; Small layer thickness (25-30 microns); Short service life; |
| Chemically cured coatings | ||
| Epoxy | Good adhesion (best due to the large number of polar groups); High mechanical strength; High solids; Large layer thickness; Excellent water resistance; Resistant to oil, petroleum products, and many solvents; High chemical resistance to aggressive gases, acids, alkalis (with short-term exposure); High durability; | Two-component, limited pot life after mixing; High requirements for climatic conditions of application. The chemical curing reaction can occur at normal speed and with high quality only at a temperature of at least +10°C; High requirements for surface preparation; Strict requirements for overcoating intervals; |
| Polyurethane | Excellent decorative properties; High weather resistance and light resistance; Excellent wear resistance and elasticity; High dry residue (for protective compounds); Large layer thickness; Higher chemical resistance to aggressive gases, acids, alkalis than epoxides; Resistance to solvents, incl. aromatic; Resistance to oil and petroleum products; Excellent water resistance; High durability; | Two-component, limited pot life after mixing; High requirements for surface preparation (for protective coatings); Toxicity upon application; |