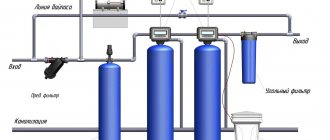
Technological production cycles of chemical, metallurgical, energy and defense enterprises use, in addition to basic materials and raw materials, ordinary water, which plays a large role in product production technology. Large volumes of fresh water used for the preparation of reagent solutions and as auxiliary cooling operations contain simply a huge amount of chemical impurities and additives that make such water dangerous even in the form of industrial wastewater.
The problem of purifying such waters, their use in a further technological cycle or discharge into the general sewer system today is completely handled by chemical wastewater treatment equipment, which ensures not only the preparation of water to the standards of household wastewater, but also even bringing purified fresh water to the standards suitable for technical use.
Basic methods of chemical treatment of industrial wastewater
Chemical methods for purifying industrial wastewater today are used mainly to bind and remove hazardous chemical elements from the volume of process water and bring the main parameters of such wastewater to standards that allow further conventional biological treatment.
Literally, in the process of such purification, the main types of chemical reactions are used:
- Neutralization of hazardous compounds and elements;
- Oxidative reaction;
- Reaction of reduction of chemical elements.
In the technological cycle of treatment facilities of industrial enterprises, chemical treatment is applicable:
- To obtain purified technical water;
- Purification of industrial wastewater from chemical compounds before discharge into the sewer system for further biological treatment;
- Extraction of valuable chemical elements for further processing;
- When carrying out post-purification of water in settling tanks for discharge into open water bodies.
Chemical treatment of wastewater before discharging it into a general sewer can significantly improve safety and speed up the biotreatment process.
Ecology DIRECTORY
The oxidation of ethylbenzene and cumene at a ratio of thermophiles to mesophiles of 1:4 proceeded 40 times slower than the oxidation of alkanes and naphthenes (see Table 4.2), and the oxidation of cyclohexane and cyclohexene was 100-125 times slower. The change in COD and BOD of wastewater containing aromatic hydrocarbons, the oxidation of sulfides to sulfates, the accumulation of carbon, as well as bacterial biomass in activated sludge occurs 1.5-2 times slower. These features of aerobic oxidation of wastewater containing arenes are associated with the specific composition of the species composition of the predominant physiological group of microorganisms (Bacterium, Bacillus, Actinomyces, Micrococcus) [10]. With a ratio of thermophiles and mesophiles of 1:8, the rate of oxidation of aromatic hydrocarbons slowed down even more, which is explained by the predominance of representatives of the genus Actinomyces in the activated sludge [10, 13].[...]
Wastewater from the production of polyamide fiber is generated at the finishing stage. The main pollutant is caprolactam, the content of which in wastewater reaches 300 mg/l, and the BOD is 630-650 mg/l. Biochemical oxidation of wastewater from this production is carried out in aeration tanks-mixers, while the organic nitrogen of caprolactam is oxidized to nitrate nitrogen. Efficiency of aeration tank operation in terms of BODtotal. is 99.4%.[...]
The degree of oxidation of organic substances depends on the contact temperature (Fig. 29, a). As can be seen from the nature of the curve, at contact temperatures above 165° C, the degree of oxidation increases, reaching 100% at a temperature of 300° C. The coefficient of excess air during the oxidation of wastewater from the production of polyvinyl chloride and copolymers is established by COD. Complete oxidation, as can be seen from Fig. 29, b, is achieved with an amount of air 4 times greater than the theoretical one. The degree of oxidation, depending on the volumetric velocity, for wastewater from the production of polyvinyl chloride was determined at an optimal contact temperature of 300° C. As can be seen from Fig. 29, c, the optimal speed can be considered 15-17 mg of wastewater per hour per 1.3 catalyst.[...]
B is the rate of wastewater oxidation; for domestic wastewater when purified to BOD2 equal to 15 mg/l, and at a temperature of 15° the value of B = 45 mg/l per hour.[...]
Temperature plays a major role in the oxidation of wastewater, with an increase in temperature the depth of oxidation increases significantly.[...]
When using technical oxygen instead of compressed air to oxidize wastewater, the permissible content of organic substances can be increased to 10%. However, the choice of oxygen or air as an oxidizing agent should be decided in each specific case based on a comparison of economic indicators. [...]
Radiation treatment (oxidation) of wastewater containing phenols, dyes, lignin and other organic substances has been tested. When exposed to cobalt gamma radiation with a dose rate of (6.8 -8) x 10 rad/h on wastewater from sulfate and sulfite cellulose production, the COD reduction was 50-80%, and at the same time neutralization of the effluent was observed: the pH value decreased from 9 to 7 .[…]
Liquid-phase thermocatalytic oxidation. Waste water after purification by secondary condensation is recommended [373, p. 45] further purification by liquid-phase oxidation using pyrolusite or regenerated manganese oxides (see Chapter 7).[...]
A method of liquid-phase oxidation of wastewater has been patented [17], which consists in the fact that wastewater enters the shshdao part of a vertically located reaction zone, which is part of a reaction device (TD), where oxygen or oxygen-containing gas is simultaneously supplied. The amount of oxygen-containing gas is calculated in such a way that the oxygen entering the reaction zone provides a 90% reduction in COD in wastewater. In the reaction zone, the temperature is maintained at 1?7-315°C and the pressure at 5.6-15.5°C. Before entering the reactor plant, wastewater passes through a counterflow tubular heat exchanger, where the temperature rises to 150-250°C due to the heat of the treated wastewater leaving the reactor plant through the same heat exchanger. The oxidation process can be single or multi-stage.[...]
The data obtained from the liquid-phase oxidation of wastewater from 5 different industries are given in Table. 7.1 [309, p. 86]. As [bottom, the liquid-phase oxidation method is highly effective and can be used to neutralize wastewater contaminated with various substances.[...]
As can be seen from Table 11, the toxicity of wastewater practically did not decrease as a result of oxidation, even despite a significant decrease in the content of total organic carbon. Obviously, the need for chemical oxidation of wastewater must be determined experimentally for each specific case.[...]
Of practical importance is the second phase of wastewater oxidation, which occurs with the participation of microorganisms in the presence of free oxygen dissolved in water, as a result of which wastewater acquires the ability not to rot. [...]
As is known, the process of chemical treatment of wastewater containing chromium compounds (Cr6+) is primarily associated with the reduction of Cr6+ to Cr3+. Since this reaction occurs in a very acidic environment (pH = 2-t-3), it is necessary to carry out appropriate oxidation of wastewater before it is carried out. [...]
Chemical oxidation of wastewater with hydrogen peroxide in the presence of a catalyst is successful. The results of this treatment of two wastewaters with different pollutant contents are shown in Table 11.[...]
We accept the specific rate of biochemical oxidation of wastewater according to table. 5.4: for the first group Pu = 6.6 mg/g-h); for the second group ri = 15 mg/g ■ h).[...]
| Graph of the progress of oxygen consumption during the oxidation of wastewater in a reservoir and the process of oxygen reduction by “re-aeration” at different water temperatures |
When using the method of vapor-phase catalytic oxidation, wastewater contaminated with volatile organic substances is fed into an evaporator, where at / 300 ° C vapors of water and organic substances are formed. These vapors, together with hot air, are fed into an evaporator loaded with a catalyst (copper-chromium, zinc-chromium, etc.), in which the process of heterogeneous catalytic oxidation of organic vapors with atmospheric oxygen occurs. The degree of neutralization reaches 99.8%. Installations for processing large volumes of wastewater have been developed. The disadvantages of the method include the possibility of poisoning the catalysts with phosphorus, fluorine and sulfur compounds. To avoid this, they must first be removed from wastewater.[...]
The Tambov plant is mastering a scheme for preliminary oxidation of wastewater with air on a nozzle made of Chiatura pyrolusite in an acidic environment, which significantly reduces the solubility of a number of organic compounds (amines, phenols), improves their sorption and reduces the consumption of active carbon. It should be noted that in an acidic environment the catalyst (manganese peroxide) is practically not poisoned.[...]
The simultaneous process of oxygen consumption during the oxidation of wastewater, which gradually fades as the mineralization of organic substances, and the process of reaeration of oxygen from the air create certain conditions for the existence of a special oxygen regime in the reservoir. It is characterized by the so-called “oxygen deflection curve” shown in Fig. 173.[...]
There are different parameters that characterize the degree of water pollution, including: chemical oxygen demand (COD) - the amount of oxygen required for complete chemical oxidation of wastewater, i.e., the amount of oxygen required to oxidize all reducing agents contained in the water. Biological oxygen demand (BOD) is the amount of oxygen required for complete biological oxidation of contaminants contained in wastewater, an indicator of water pollution. [...]
| Absorption isotherms of organic substances (products of biological oxidation of wastewater) by active carbon of the KAD-iodine grade from wastewater from isoprene production (curve 1) and phenolic (curve 2). |
Starting from a concentration of 1.0 mg/l, nickel reduces the efficiency of biological wastewater treatment, weakens the intensity of activated sludge nitrification processes, and significantly reduces the aerobic oxidation of wastewater on biological filters; at a concentration of 2.0 mg/l, nickel has a toxic effect on the microflora of digesters and inhibits sludge fermentation.[...]
The mode for measuring the rate of oxygen consumption is selected in such a way that the process of wastewater oxidation occurs with a lack of organic contaminants. In this measurement mode, corresponding to the initial portion of the curve described by a Monod-type equation, a close to linear relationship is observed between the MIC and the rate of oxygen consumption. The values of the coefficients in this dependence are preserved only if the characteristics of activated sludge and wastewater remain unchanged. However, in real conditions of biological treatment, both the composition and concentration of organic pollutants in wastewater change, and the properties of activated sludge do not remain constant. For these reasons, it is impossible to obtain reproducible results when determining the MIC from the rate of oxygen consumption only by measurements in the test water. [...]
There is great interest in the development and application of heterogeneous catalytic systems for the oxidation of wastewater with hydrogen peroxide.[...]
Flocculants with powder additives are used for high-gradient magnetic separation of natural and waste waters after the addition of magnetite, for the catalytic oxidation of waste water on the surface of a sorbent - catalyst, for deodorization and decolorization of natural waters with active carbon, as well as for the treatment of water with clay minerals in order to extract radioactive contaminants and other impurities [117].[...]
In those same years, based on laboratory research by GIAP [15J], an industrial plant (with a capacity of 2 m3/h) was designed for the oxidation of wastewater from the production of caprolactam at the Lisichansk Chemical Plant. Built in 1962, the installation produced good results throughout its entire period of operation using wastewater from one of the plant’s chemical production facilities.[...]
The term “activated sludge” was first coined in 1914. An article was published in the Society of the Chemical Industry magazine reporting the results of experiments on wastewater treatment without the use of filters. The authors of the article carried out a series of experiments at wastewater treatment plants in Manchester and proposed a new treatment method that could reduce the time required to oxidize wastewater to several hours. The essence of the method was that wastewater was mixed with sludge formed as a result of preliminary oxidation of water. This method is called “activated sludge treatment.” [...]
Nitrogen and phosphorus have a beneficial effect on the development of microorganisms. These two components are necessary for the normal development of biomass. The lack of these substances in water leads to a sharp decrease in the physiological activity of microorganisms and the intensity of wastewater oxidation. The consumption of nutrients depends on the increase in biomass, which in turn depends on the class of the oxidized substance, the type of microorganisms, the phase of their development and other factors. [...]
The difference between COD and BOD characterizes the presence of impurities that are not oxidized biochemically and the amount of organic substances used to build microbial cells. For domestic wastewater, BODtotal is 85-90% of COD. Based on the BODtotal/COD ratio, one can judge the possibility of using a certain method of wastewater treatment. If the BOD/COD ratio is >0.5, then this indicates the possibility of using biochemical wastewater treatment; at the BOD/COD ratio[...]
From a physicochemical point of view, activated sludge is a structured colloidal system with a high sorption capacity. Activated sludge is a habitat for many water and soil microorganisms that form a complex biocenosis. It is obtained by long-term aeration of the biological film of biofilters or domestic wastewater. The composition of activated sludge is determined by the nature of organic impurities and therefore can change qualitatively and quantitatively. Thus, during the oxidation of phenol-containing wastewater, pseudomonas (Pseudomonas) predominate in the activated sludge biocenosis, with a total number of microorganism species of 48 and a bacterial population density of 550 million cells per 1 ml. And during the oxidation of wastewater containing anionic surfactants (alkyl sulfonates), only ten species develop with a significant predominance of mycobacteria with a total number of microorganisms of 500,000 in 1 ml. [...]
However, without the addition of nutrients, the process of biological decomposition does not proceed completely, which actually explains the limited success that Bode achieved [5] in his experiments. He proposed to carry out the oxidation of wastewater in treatment ponds with a large water surface and shallow depth, as is the case when processing wastewater from distilleries, etc. [eleven].[ …]
The change in the rate of oxygen consumption along the length of the aeration tank for each of the modifications and the corresponding change in the concentration of dissolved oxygen (with a known distribution of aeration intensity) can be detected by calculation (with a known law and rate of wastewater oxidation and a known hydrodynamic model of flows) or experimentally (based on averaging of profiles concentrations of dissolved oxygen measured in different aeration tanks).[...]
Neutralization of industrial wastes
Most industrial enterprises using chemical treatment of industrial wastewater most often use in their treatment plants and complexes means to neutralize the acidic and alkaline indicators of water to an acidity level of 6.5–8.5 (pH) acceptable for further processing. A decrease or, conversely, an increase in the acidity level of wastewater allows the liquid to be further used for technological processes, since this indicator is no longer dangerous to humans.
Water brought to this level can be used for the technological needs of enterprises, in auxiliary production, or for further purification using biological agents.
It is important that the chemical normalization of water carried out at enterprises effectively ensured the neutralization of acids and alkalis dissolved in wastewater and prevented them from entering the soil and aquifers.
Exceeding the amount of acids and alkalis in discharged waste leads to accelerated aging of equipment, corrosion of metal pipelines and shut-off valves, cracking and destruction of reinforced concrete structures of filtering and treatment stations.
In the future, to normalize the acid-base balance of waste in settling tanks, tanks and filtration fields, more time is needed to carry out biological treatment, 25-50% more time than neutralized wastewater.
Industrial technologies for neutralization of liquid waste
Carrying out chemical treatment of liquid waste using the neutralization method is associated with leveling the required acidity level of a certain volume of wastewater. The main technological processes involved in neutralization are:
- determination of the level of pollution by chemical compounds in wastewater;
- calculation of the dosage of chemical reagents required for neutralization;
- clarification of water to the required level of standards for liquid waste.
The selection of treatment equipment, its location, connection and operation depends, first of all, on the level of pollution and the required volumes of waste treatment.
In some cases, mobile chemical treatment units are sufficient for this purpose, providing cleaning and neutralization of a relatively small amount of liquid from the enterprise’s storage tank. And in some cases, the use of a permanent chemical cleaning and neutralization installation is required.
The main type of technological equipment for such stations is flow cleaning or contact type installations. Both installations allow you to provide:
- pollution control;
- the possibility of using a scheme for mutual neutralization of acidic and alkaline components in the technology;
- the possibility of using the natural neutralization process in technological reservoirs.
Technological schemes for chemical cleaning using the neutralization method must provide the ability to remove or remove solid, insoluble sediment particles from treatment tanks.
The second important aspect of the operation of treatment plants is the ability to timely adjust the required quantity and concentration of reagents for the reaction, depending on the level of contamination.
Typically, the technological cycle uses equipment that has several storage tanks to ensure timely reception, storage, mixing and discharge of wastewater brought to the required condition.
How to neutralize wastewater
Neutralization of wastewater helps to normalize the pH value. This chemical composition of water is not dangerous for humans and nature. It can be reused for various needs.
The neutralization process is based on the use of reagents that are used taking into account the concentration and constituent elements of the acidic environment. Experts distinguish 3 types of wastewater with acids:
- predominance of weak acids;
- presence of strong acids;
- predominance of sulfuric and sulfurous acid.
Neutralization of waters with sulfuric acid depends on the reagent used. The process occurs at different levels. If you use lime milk, then gypsum will fall out as a residue. It will settle on the walls of the pipes.
To neutralize alkaline waters, acids or acidic gases are used. Using the latest technology, simultaneous neutralization of wastewater and purification of harmful gas components is carried out. To calculate the amount of acid gas required, the level of mass transfer is determined. This technology is considered resource-saving, since it eliminates the discharge of wastewater, reducing the consumption of fresh water, saving thermal energy for heating it.
When developing a technological scheme for wastewater neutralization, the following is taken into account:
- possible simultaneous neutralization of alkalis and acids entering with wastewater;
- presence of alkaline reserve;
- natural neutralization of water bodies.
To implement the process under consideration, special equipment is used. Neutralization is carried out in a storage tank, settling tank or illuminator. The choice of equipment depends on climatic conditions and the duration of wastewater storage.
To implement neutralization, various chemicals are added to the wastewater, which react with acids or alkalis to form a suspension. It precipitates. Its volume is determined by the following indicators:
- the amount of metals, acid ions in the source water;
- quantity and water of the reagent used;
- the level of lightening used.
Chemical neutralization of wastewater by mixing acidic and alkaline components
Using the method of neutralizing wastewater by mixing acidic and alkaline components allows for a controlled neutralization reaction without the use of additional reagents and chemicals. Controlling the amount of wastewater discharged with acidic and alkaline compositions allows for timely operations to accumulate both components and dosage during mixing. Typically, for continuous operation of treatment facilities of this type, a daily volume of discharge is used. Each type of waste is checked and, if necessary, brought to the required concentration by adding a volume of water or determining the volume proportion for the purification reaction. Directly at the treatment plant, this is carried out in the station’s storage and control tanks. The use of this method requires the correct chemical analysis of the acid and alkaline components, carrying out a salvo or multi-stage neutralization reaction. For small enterprises, the use of this method can be carried out both in local treatment facilities of a workshop or site, and with the help of treatment facilities of the enterprise as a whole.
Purification by adding reagents
The method of purifying liquid waste with reagents is used mainly for purifying water containing a large amount of one type of contaminant, when the normal ratio of the alkaline and acidic components in the water is significantly in one direction.
Most often, this is necessary when the contamination has a pronounced appearance and cleaning by mixing does not give results or is simply irrational due to the increased concentration. The only and most reliable method of neutralization in this case is the method of adding reagents - chemicals that enter into a chemical reaction.
In modern technologies, this method is most often used for acidic wastewater. The simplest and most effective method of neutralizing acid is usually to use local chemicals and materials. The simplicity and effectiveness of the method lies in the fact that waste, for example, from blast furnace production, perfectly neutralizes sulfuric acid pollution, and slag from thermal power plants and power plants is often used to add to tanks with acid discharges.
The use of local materials can significantly reduce the cost of the cleaning process, because slag, chalk, limestone, and dolomite rocks perfectly neutralize large amounts of heavily contaminated wastewater.
Waste from blast furnace production and slag from thermal power plants and power plants does not require additional preparation other than grinding; the porous structure and the presence of calcium, silicon and magnesium compounds in the composition allow the use of materials without pre-treatment.
Chalk, limestone and dolomite used as reagents must undergo preparation and grinding. In addition, for cleaning, some technological cycles use the preparation of liquid reagents, for example, using lime and an ammonia water solution. In the future, the ammonia component greatly helps in the process of biological water purification.
Application of reagents
Neutralization with reagents is used if the balance between acid and alkali is disturbed in the wastewater. In such cases, the possibility of implementing the process in question by mixing water is excluded. To solve the problem, missing chemicals are added to the wastewater. This technology is most often used in the presence of acidic waters.
Their neutralization is based on the use of waste from various industries (sludge that is formed after chemical cleaning at thermal power plants). In the presence of sulfuric acid, steelmaking slags are used.
The effectiveness of this technology is based on the presence in them of a large amount of magnesium and calcium oxide compounds. The following data is taken into account:
- the amount of calcium salts inherent in water and capable of dissolving well;
- the amount of calcium salts that are poorly soluble in water.
Lime is introduced into drains in the form of milk or dry powder. The most economical option is to use fluff lime. If it is necessary to process up to 200 cubic meters. water, then use soda.
Wastewater oxidation method
The wastewater oxidation method makes it possible to obtain wastewater that is safe in its toxicity characteristics in hazardous chemical industries. Most often, oxidation is used to produce effluents that do not require further solids extraction and can be discharged into the public sewer system. Chlorine-based oxidizers are used as additives; this is the most popular cleaning material today.
Materials based on chlorine, sodium and calcium, ozone and hydrogen peroxide are used in multi-stage wastewater treatment technology, in which each new stage significantly reduces toxicity by binding hazardous toxic substances into insoluble compounds.
Oxidation plants with multi-stage purification systems make this process relatively safe, but the use of toxic oxidizers such as chlorine is gradually being replaced by safer, but no less effective methods of waste oxidation.
Determination of BOD and COD
BOD or biochemical oxygen demand determines the amount of oxygen that aerobic microorganisms will need to decompose easily oxidized organic matter. This process can be described as the oxidation of organic matter by biological means, resulting in the formation of carbon dioxide and water. In practice, the values most often used are BOD5 and BODtotal, which are expressed as the amount of O2 in mg per liter.
COD (chemical oxygen demand) characterizes the generalized amount of oxygen required to chemically oxidize organic compounds contained in wastewater. In other words, it allows you to determine the required amount of oxygen that will be required to convert organic carbon into carbon dioxide, nitrogen-containing substances into ammonia, and sulfur-containing substances into sulfur dioxide.