Galvanizing electrolytes. Classification and types
Galvanizing electrolytes, according to the nature of the medium, are divided into: acidic (chloride, sulfate, hydrofluorosilicic, hydrofluoride) weakly acidic electrolytes (sulfate-ammonium, ammonium chloride, ammonium-free chloride), weakly alkaline or neutral (ammonium, pyrophosphate, amino complex), alkaline electrolytes (cyanide , zincate).
Electrolytes for galvanizing can be simple in composition (these include acidic and weakly acidic electrolytes), complex.
In simple galvanizing electrolytes, the metal is in the form of cations (Zn^(2+)*mH_2 O). The mechanism of zinc discharge at the cathode is based on the reaction of adding two electrons to each zinc ion: Zn^(2+)*mH_2 O+2¯e=Zn+mH_2 O. Divalent zinc ions are discharged at low cathodic polarization (20-40 mV). Current efficiency in simple electrolytes reaches 98%. The dissipative ability of simple electrolytes is low. The structure of the deposited layer has an uneven, coarse-crystalline structure.
Complex galvanizing electrolytes are characterized by the presence of complex zinc anions in the working solution: [〖Zn(CN)〗_4 ]^(2-) , [〖Zn(OH)〗_4 ]^(2-), [〖Zn(CN)〗 _4 ]^(2-). The mechanism of zinc ion discharge can occur in two ways:
- [Zn〖(CN)〗_4 ]^(2- )= Zn^(2+)+ 4CN^-; Zn^(2+)+2¯e=Zn.
- [Zn〖(CN)〗_4 ]^(2-)+2¯e=Zn+4CN^-.
Zinc deposition on the cathode (discharge) occurs at high cathodic polarization. The current efficiency in complex electrolytes of different chemical compositions is significantly reduced compared to simple ones. Scattering power increases. The microstructure of the zinc coating has a fine-grained structure.
Technological process "ZINKAT"
High speed matt galvanizing
for painting or phosphating
- The process can be used in both conventional lines and high-speed overhead installations for the application of matte zinc coatings
- Coatings are ideal underlayer for subsequent paint coatings
: powder, cataphoresis, etc.
- Zinc coatings can be phosphated before painting.
- The formed zinc coatings are characterized by high plasticity, which makes it possible to obtain thick layers of zinc, up to 20–35 microns
- Deposition is carried out at high current densities, 8-10 A/dm2
, which makes it possible to obtain a thickness of
25 microns
in approximately
25 minutes - The process is characterized by high values of covering and dissipation power at low current densities
- The electrolyte is not afraid of overheating, the quality is maintained up to 50 ºС and above
- Electroplating at thickness ~25 µm
can successfully compete with thicker hot-process coatings
| Chemicals and additives are required to prepare 1000 liters of electrolyte | Process parameters | |||
| Zinc oxide, ZnO | 23 kg | Interval | Optimum | |
| Caustic soda, NaOH | 142 kg | Zinc (Zn2+) | 15–22.5 g/l | 20 g/l |
| Basic additive " ZINCAT " | 4 l | NaOH | 120–150 g/l | 140 g/l |
| Aux. additive "Cleaner" | 1 l | Temperature | 25–50 ºС | 30ºС |
Approximate consumption: a) 1 liter of ZINCAT
» at 16000–26500 A∙h;
Cleaner” is consumed from 1 liter of the main additive
».
See also other galvanizing processes from
LLC "SONIS"
Galvanizing - instead of hot-dip galvanizing
Electrolytic zinc coatings are unfairly little used to protect steel products under particularly harsh operating conditions.
.
Even experts lose sight of the fact that with a thickness of ~25 microns,
zinc galvanic coatings are comparable in their protective ability to thicker, but extremely heterogeneous in structure, composition and thickness (typical range from 30 to 100 microns) coatings obtained by hot-dip galvanizing.
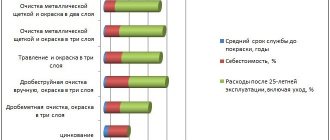
The graph shows the results of corrosion tests under the same conditions of electroplating and “hot-dip galvanizing”. As you can see, the loss of zinc mass due to corrosion over time is significantly higher for “hot” coatings than for electrolytic ones. The high protective ability of the latter is explained by the fact that they are chemically purer and homogeneous in phase composition, have a finer-grained and dense structure - all this significantly inhibits corrosion processes.
At the same time, galvanic shops in which routine work is the application of relatively thick (18 microns and above) zinc coatings are very rare. The main reasons: a) thick coatings are prone to cracking due to increased internal stresses; b) the productivity of the process is low - galvanizing takes a lot of time; c) when galvanizing complex-profiled products, due to the low dissipative ability of the electrolyte, the coating is too uneven in thickness.
However, all these problems are eliminated if you use the ZINKAT technological process. The composition of the electrolyte is simple: 20 g/l ZnO, 135 g/l NaOH and 4 ml/l ZINKAT additive. The process is specifically designed for high-performance matt galvanizing in suspension lines. Thus, at a current density of 8–10 A/dm2, zinc with a thickness of 25 microns is deposited in 25 minutes. The electrolyte is characterized by excellent dissipation ability. Special ingredients in the additive ensure high plasticity of coatings, which eliminates their “bubbling” and cracking even with a thickness of 35 microns and above. At the final stage, the coating can be passivated or phosphated in a standard manner. If galvanized products are painted, then the galvanic coating deposited in zincate electrolytes serves as an ideal sublayer for paint and varnish coatings (including powder). Due to the columnar structure of sediments, their surface is characterized by a developed microrelief, which significantly enhances the adhesion of paintwork.
In Russia, there are examples where instead of “hot-dip galvanizing”, galvanic coatings deposited on steel from zincate electrolyte with the addition of “ZINCAT” are successfully used. We are talking about galvanizing elements of steel fences with subsequent painting (double protection system). As many years of observations have shown, galvanic coatings perfectly protect steel structures installed on the street from corrosion, in no way inferior to “hot-dip galvanized.”
Thus, for applying protective zinc coatings to steel products operating under particularly harsh conditions
, if necessary, instead of hot-dip galvanizing, the more affordable galvanic galvanizing can be successfully used.
Main technological parameters of electrolytes for galvanizing
The main distinguishing features of galvanizing in various electrolytes are: · cathodic polarization - a change in the discharge at the cathode when the current density changes (with high cathodic polarization, the electrolyte has high electrical conductivity), · polarizability of the electrolyte during the process of zinc deposition (the higher the polarizability (potential) when the current density changes , the better the dispersing ability of the electrolyte), · kinetics and mechanism of zinc electrodeposition.
The criteria for polarization and polarizability are actively influenced not only by the type of electrolyte (simple, complex), but also by the operating temperature range during galvanizing, chemical composition, concentration of constituent components, and brightening active additives. The highest polarizability rates are inherent in alkaline electrolytes (cyanide, zincate).
The magnitude of the discharge of complex ions at the cathode is higher than the cathodic polarization of hydrated ions. Consequently, the dispersing ability increases, the structure of the surface layer becomes uniformly fine-grained and dense. However, the current output decreases. The zinc at the anode dissolves through a galvanic process to form positively charged hydrated ions and complex anions.
Comparative analysis of electrolytes for galvanizing
Zinc salts in acidic and weakly acidic electrolytes are highly soluble in water, so anodic dissolution of zinc occurs easily, without difficulty and practically without the release of hydrogen. The process occurs at low cathodic polarization, with high current efficiency and deposition rate, and low dissipation ability.
The main advantages of galvanizing in acid electrolytes include: · high productivity of the process, · stability and simplicity, · low percentage of hydrogenation of the finished product, · absence of toxic substances in the production process. Acidic electrolytic solutions are used for galvanizing parts of a simple configuration (wire, sheets, tape, rods, plates).
In alkaline electrolytic solutions, anodic - cathodic processes are difficult. This is explained by the fact that · the release of zinc at the cathode occurs from complex compounds (in an alkaline environment, hydrolysis of zinc salts occurs with the formation of an insoluble precipitate, therefore, the working solution requires components that are capable of forming water-soluble zinc compounds), · the discharge of zinc is accompanied by the release of hydrogen (when increasing the current density, the potential for zinc release at the cathode increases and the rate of hydrogen release increases), · the composition of the working solution contains various contaminants and impurities, · there is the possibility of both electrochemical and chemical reactions of zinc dissolution occurring at the anode. All of the above factors reduce the metal yield by current.
In alkaline electrolytes , due to high cathodic polarization, the deposited zinc is uniformly distributed on the cathode surface, therefore they are used for galvanizing products of various geometric shapes and configurations. The disadvantages of alkaline galvanizing include the following indicators: · hydrogenation of the galvanized surface, which worsens the mechanical properties of the product, reduces ductility, increases the possibility of brittle fracture, · instability of the chemical composition, · the presence of toxic substances (cyanide) in the composition of some electrolytes.
When choosing the type of electrolyte for industrial use, the following are taken into account: · productivity, · technological ease of use, · the possibility of obtaining a uniform coating, · thickness and structure of the surface layer, · coating of parts of various configurations, · environmental parameters. A variety of electrolytic compositions expand the possibilities for applying zinc plating by electroplating.
Features of the anodic process in various galvanizing electrolytes
More about galvanizing >>
hide
- Companies offering galvanizing
- Book “Galvanizing. Equipment and technology"
- Galvanizing of contaminated threaded products
- Features of the anodic process in various galvanizing electrolytes
- Behavior of anodes in various galvanizing electrolytes
- Method for determining the current output (CT) of zinc
back forward
For technologists
To obtain high-quality zinc coatings, it is necessary to comply with all the recommendations specified in the technological instructions and regulations of the enterprise - from the incoming inspection of parts arriving for galvanic processing to the storage of finished products. Naturally, failure to comply with the requirements for the anodic process, which is an integral part of the entire technology, can have a decisive impact on the quality of the resulting coatings. As in other galvanic technologies, anodic processes when galvanizing parts from various types of electrolytes have their own characteristics, which should be taken into account when designing the main technological and auxiliary equipment, as well as when operating electrolytes.
Let us consider some features of the anodic processes of the three most common types of galvanizing electrolytes in industry - cyanide, alkaline zincate and slightly acidic, the typical compositions of which are given in Table 1. In general, the basic requirements for the operation of soluble anodes can be formulated as follows. Anodes must:
- work in a stable soluble state, without the formation of salt and other passivating films on the surface;
- ensure during operation and downtime of the electrolyte the concentration of metal ions in it in the optimal range;
- dissolve evenly over the surface, minimizing chipping (slagging) of the metal;
- due to the geometric dimensions, configuration and location relative to the parts being coated, promote the most uniform distribution of the deposited metal on the surface of the parts in terms of thickness.
To maintain a constant concentration of zinc ions in the electrolyte, it is necessary that the rate of entry of metal ions into the electrolyte due to the dissolution of the anodes compensates for the rate of its consumption for coating deposition and for the carryover of the electrolyte with parts and on technological equipment (suspensions, drums, bells).
Table 1. Compositions of some typical galvanizing electrolytes
| Component, g/l | Electrolyte No. 1 | Electrolyte No. 2 | Electrolyte No. 3 |
| Zinc chloride | — | — | 60-100 |
| Ammonium chloride | — | — | 200-220 |
| Boric acid | — | — | 10-20 |
| Zinc oxide | 25-30 | 10-12 | — |
| Sodium hydroxide | 60-90 | 100-120 | — |
| Sodium cyanide (general) | 30-50 | — | — |
| Temperature, °C | 18-30 | 18-30 | 18-30 |
| pH | — | — | 4,5-5,5 |
| Cathode current density, A/dm2 | 0,5-3,5 | 0,5-3 | 0,5-1,5 |
The compositions of electrolytes are given without special shine-forming and other additives.
The peculiarity of the operation of zinc anodes is associated with the low thermodynamic stability of zinc metal in corrosive environments, which include all galvanizing electrolytes. As a result, zinc dissolves (corrodes) when immersed in an electrolyte, even in the absence of anode current. Since this process occurs spontaneously, it is almost impossible to reduce its speed in the conditions of existing production. Self-dissolution rate
zinc concentration does not depend on the value of the anodic current density, but depends on the pH, temperature of the electrolyte, as well as on the nature and concentration of the substances included in the solution. This property of zinc metal leads to a gradual accumulation of zinc ions in solution, which is undesirable.
The rate of electrochemical (under the influence of current) dissolution depends on the anodic current density, pH, temperature and composition of the electrolyte. Obviously, the closer the values of the cathode (VTk) and anodic (VTa) current outputs, the easier it will be to maintain the required concentration of zinc ions in the electrolyte.
The simplest, from this point of view, to operate are weakly acidic galvanizing electrolytes based on potassium or ammonium chloride salts, in which only soluble zinc anodes are used. In these electrolytes, VTa is almost equal to 100%, while VTk is 92-95%. Balance in the concentration of metal ions is achieved due to the removal of the electrolyte and the subsequent addition of water to the electrolyte. In production, it is not very difficult to determine the required ratio of cathode and anode surfaces for stable operation of the electrolyte. In this case, electrolytes during operation are generally not adjusted with zinc salts. The pH value of electrolytes is close to neutral (4.5-6.0) and, despite the high concentration of corrosive chlorine ions, the rate of self-dissolution of zinc anodes is very low.
In alkaline cyanide electrolytes, VTa is close to 100%. In this case, VTk in the operating range of current densities is 60-80%. Self-dissolution of zinc anodes also occurs in these electrolytes, although not at such a high rate that should correspond to the high alkalinity (pH> 13) of the solution. In many cases, maintaining the optimal zinc concentration is achieved by carrying away the electrolyte with the parts (with a large production program). However, when using cyanide electrolytes, the concentration of zinc ions should be carefully monitored and the anodes should be removed when the bath is not in use. First of all, this applies to electrolytes with a low concentration of zinc ions.
The most noticeable difference in the rates of consumption and supply of zinc ions is observed in cyanide-free alkaline zincate electrolytes. If zinc anodes operate in a stable soluble state, then VTa is almost equal to 100%. In addition, in solutions containing a high concentration of free alkali, intense self-dissolution of zinc (anodes) occurs. Considering that VTk is usually 50-70%, it is obvious that it is very difficult to maintain the concentration of zinc ions in a relatively narrow range (for example, 6-10 g/l) without the use of special technical solutions. Therefore, during the operation of zincate electrolytes, the concentration of zinc in them constantly increases. It should be noted that sometimes in automatic lines with high productivity when processing complex-profile parts on hangers or small parts in bulk in drums, stabilization of the zinc content is achieved due to large carryover of the solution and subsequent addition of water to the bath.
The above-listed features of the anodic behavior of zinc anodes in various galvanizing electrolytes determine the specifics of the organization and control of the anodic process, as well as possible problems that arise during operation.
One of the most common problems when working with soluble anodes is salt passivation of their surface. When the anode is dissolved, the concentration of metal ions in the electrolyte layer directly near the anode surface is almost always higher than in the bulk of the electrolyte. The resulting zinc ions (in the general case Zn→Zn2+ + 2 e-
) should be removed (diffuse) from the surface of the electrode deep into the solution. If for some reason an increased anodic current density is realized during the electrolysis process, the rate of formation of zinc ions can be significantly higher than the rate of their diffusion from the anode into the solution. In this case, the concentration of metal ions in the near-anode layer of the solution increases and after some time the maximum solubility of zinc salts is reached. Some of the resulting finely dispersed solid particles of zinc compounds (salts) are adsorbed on the surface of the anode. As a result, after some time a dense salt film forms on the surface of the anode, which impedes the ionization of zinc, i.e. The surface of the zinc anode, which is active at the beginning of electrolysis, passes into a passive (insoluble) state.
For weakly acidic electrolytes, this phenomenon is relatively rare. This is due to the fact that the solubility of zinc chloride or sulfate salts is very high. In addition, in these electrolytes, zinc, as a rule, is in the form of simple (hydrated) ions, the mobility of which, and, consequently, the rate of diffusion (removal) from the anode surface is high.
In alkaline cyanide and zincate electrolytes, the possibility of the formation of passive salt films is much higher. Firstly, this is due to the fact that the resulting zinc ions first form a soluble stable complex, for example Zn(CN)42- or Zn(OH)42-, and only then are removed into the depth of the solution. Secondly, the solubility of complex zinc salts such as Na2[Zn(CN)4] or Na2[Zn(OH)4] is significantly lower than that of simple salts. In addition, the mobility of large complex anions is also low. All this determines the high probability of the formation of insoluble salt films on the surface of zinc anodes in these electrolytes.
The solubility of complex metal salts depends not only on the concentrations of the metal ion and the complexing reagent (ligand), but also on the ratio of their concentrations in solution. In other words, complex salts can exist in a soluble state only in the presence of a certain excess of the complexing reagent.
In cyanide electrolytes, the normal operation of zinc anodes depends on the concentration of OH- and, to a slightly lesser extent, on the concentration of CN-. Therefore, if the concentration of these complexing components is insufficient, a dense salt film, consisting mainly of insoluble zinc hydroxide compounds, quickly forms on the surface of the zinc anode.
In alkaline zincate electrolytes, for the normal occurrence of both cathodic and anodic processes, the ZnO/NaOH concentration ratio is recommended to be maintained in the range from 1:8 to 1:12 (optimally 1:10). When the alkali concentration is insufficient, part of the resulting zinc ions in the near-anode layer of the electrolyte forms insoluble forms of hydroxide compounds, which form a dense passive film on the surface of the anode.
Thus, in complex alkaline galvanizing electrolytes it is necessary to carefully maintain not only a certain concentration of each component, but also their ratio in the solution. For example, if the concentration of NaOH in the zincate electrolyte is in the optimal range, and the concentration of zinc ions has increased during operation, then the ratio of their concentrations has changed. As a result, the near-anode layer of the electrolyte begins to lack OH- ions to dissolve the resulting zincate complex, and the anode surface is gradually covered with a passive film.
The formation of salt passivation films on zinc anodes can also be caused by too high anode current density. In this case, the rate of formation of zinc ions can be so high that, despite the optimal concentrations of complexing substances and metal in the bulk of alkaline electrolytes or the high solubility of salts in weakly acidic electrolytes, insoluble compounds will form in the anode layer of the electrolyte, passivating the anode surface.
Another reason for salt passivation of zinc anodes is the accumulation of carbonates in alkaline electrolytes coming from the air. At a carbonate concentration of 60-80 g/l (which, by the way, at this concentration degrades the quality of zinc coatings), crystallization of sodium and zinc carbonate compounds of various compositions on the anode surface is possible.
It should be noted that salt passivation of even a small part of the anode surface is very dangerous for the normal implementation of the technological process and the production of high-quality zinc coatings. In this case, the active surface decreases (on which normal dissolution of the metal occurs). Since the current strength in the bath does not change during operation, a decrease in the actively dissolving anode surface leads to an increase in the anodic current density. The rate of metal dissolution begins to exceed the rate of removal of ions deeper into the solution, and additional conditions are created for the formation of insoluble metal compounds and passivation of the surface.
Passivation of soluble anodes leads not only to a decrease in the rate of entry of zinc ions into the electrolyte and a violation of its optimal concentration. Since the salt film is not a conductor, voltage begins to increase on bathtubs (especially drum and bell types). At the same time, due to the high resistance, part of the electricity is spent on the formation of thermal energy and the electrolytes begin to heat up. An increase in the temperature of most galvanizing electrolytes by more than 30-35 ° C leads to a deterioration in the quality (brilliance) of coatings and a decrease in the dissipative ability of electrolytes on metal.
Another problem is that the resulting finely dispersed insoluble compounds are not only adsorbed on the surface of the anode, but also, being in the volume of the electrolyte, can be included in the zinc coating on parts. Fine particles do not settle to the bottom for a very long time. At the same time, not only the decorative, but also the basic functional properties of zinc coatings—the protection of steel from corrosion—deteriorate. In particular, a passive film does not form on the surface of non-metallic inclusions in chromate or phosphate solutions, its porosity increases, corrosion resistance and protective effect decreases.
An increased anodic current density leads not only to salt passivation of the anodes. It is known that anodic dissolution of metals occurs unevenly over the surface. The most intense dissolution occurs along the boundaries of metal grains. The uniformity of dissolution is also influenced by the chemical composition of the anodes (impurities of other metals) and the microgeometry (roughness) of the anode surface and other factors. The uneven rate of dissolution leads to the fact that individual small pieces of metal, without having time to dissolve, fall out of the metal structure. This is called spalling or slagging of the anodes. Large particles quickly settle to the bottom of the bath, while very small ones can remain suspended in the solution for a long time and be included in the cathode coating. This often manifests itself in the fact that the surface of the zinc coating becomes rough, although shiny. Anode chipping leads to an increase in unproductive losses of non-ferrous metal.
Of course, to prevent solid particles from entering the electrolyte, you can use anode covers made of chemically resistant (preferably polypropylene) fabric. Naturally, after some time the covers become clogged with sludge and must be periodically cleaned or replaced. Otherwise, the electrical resistance will increase and the voltage on the galvanic bath will increase. In this regard, it is necessary to emphasize the special role of filter units, which constantly or periodically clean electrolytes from inevitably accumulating mechanical impurities. In addition, with continuous filtration (2-3 volumes per hour), the electrolyte is mixed. Stirring, having a beneficial effect on the cathode process, also helps to accelerate the removal of the resulting zinc ions from the surface of the anodes, reducing the likelihood of the formation of insoluble compounds.
An important technological indicator is the ratio of the cathode and anode surfaces in the galvanic bath. In the case of using only soluble anodes, their working surface is determined, first of all, by the permissible value of the anodic current density at which zinc operates in a stable soluble state. When calculating the required current strength for deposition of high-quality zinc coatings, it should be borne in mind that the side of the anode plate facing the bath wall acts as a soluble anode on no more than 30% of this surface.
As a rule, for all galvanizing electrolytes, the ratio between the surface to be coated and the surface of the soluble anodes (Sk:Sa) is recommended in the range from 1:1 to 1:1.5.
At the same time, the most stable operation of zinc anodes in weakly acidic and alkaline electrolytes corresponds to 1-1.5 A/dm2. If the estimated surface area of the anodes is large and they cannot be placed in a galvanic bath, bulk anodes are used in the form of zinc plates cut into cubes or, more preferably, in the form of balls. They are poured into corrugated titanium baskets and placed on anode rods. The dimensions and number of titanium baskets are determined by the geometric dimensions of the bath, the type and size of galvanic equipment (suspensions, drums, etc.), and the configuration of parts. Bulk anodes are often used in drum and bell type baths, in which the surface area of the parts being processed is large.
A special case of organizing the anodic process occurs for alkaline zincate electrolytes. Since it is difficult to ensure a constant concentration of zinc in these electrolytes during operation of the electrolytes, to maintain the balance it is necessary to use either only insoluble anodes, or replace some of the zinc anodes with insoluble ones. The most suitable material for these purposes is low-carbon steel (for example, St3) or nickel. Sometimes a nickel coating with a thickness of 15-20 microns is applied to steel plates by galvanic method. This is due to the fact that carbon steel and nickel are well passivated in alkaline media and do not dissolve even when used as an anode. The use of high-alloy chromium-nickel steels is not recommended, since during operation chromium dissolves and the electrolyte becomes contaminated. Purification of Cr(VI) ions from galvanizing electrolytes is very difficult. During electrolysis on insoluble anodes, an oxygen evolution reaction occurs:
H2O → O2 + 2e-
Insoluble anodes in alkaline galvanizing electrolytes can be used in various ways. Only insoluble anodes can be used. In this case, it is necessary to adjust the electrolyte for zinc very often. To make adjustments, as a rule, a certain volume of electrolyte is taken and the calculated amount of zinc oxide (ZnO) is dissolved in it. It is possible, if the consumption of other components allows, to dissolve ZnO in a NaOH solution in a separate container and then add it to the electrolyte. However, these methods are not always convenient, since they require accurate calculation of the amount of ZnO that can be dissolved in the electrolyte without the formation of insoluble compounds.
Often zinc oxide powder is poured directly into the galvanizing bath. It should be borne in mind that with this method of adjustment, part of the powder does not dissolve and is present in the electrolyte in the form of suspended particles. After such adjustments, filtration of the solution is required, but even in this case, the quality of the coatings, as a rule, deteriorates, and the electrolyte must be processed under current to restore its properties.
A fairly common use for insoluble anodes is to periodically hang them (instead of soluble anodes). After some time, when the concentration of zinc ions decreases to the required value, a reverse replacement is performed. It is also possible to use soluble and insoluble anodes simultaneously. The ratio of the surface of the cathode and anodes remains from 1:1 to 1:1.5. The ratio of the surface area of soluble and insoluble anodes is selected for each production depending on the operating conditions of the electrolytes and on the basis of systematic chemical analysis data that determines the rate of supply and consumption of zinc in the electrolyte.
In cyanide galvanizing electrolytes, the use of insoluble anodes is recommended only in cases of extreme necessity, when other measures do not prevent the accumulation of zinc in the electrolyte. This is due to the fact that on insoluble anodes, destruction (oxidation) of one of the main components of the electrolyte—cyanide ion—occurs. Therefore, when working with insoluble anodes, the NaCN content in the electrolyte should be monitored and adjusted more often. The use of insoluble anodes is advisable for the selective purification of electrolytes from impurity metals by processing at low current densities.
During the electrodeposition of zinc from an alkaline zincate electrolyte on insoluble anodes, intense release of oxygen gas occurs. Considering that hydrogen is released at the cathode (BTZn ~ 60-80%), a large number of aerosols containing a high concentration of alkali are formed in the working area of the galvanic bath above the solution. In this case, to ensure normal working conditions, it is necessary to increase the air suction rate through the on-board ventilation. It is also necessary to check the compliance of the performance of filter elements that purify acid-base air emissions with the increased removal of pollutants into the atmosphere.
Currently, for alkaline zincate electrolytes, it is more often proposed to use only insoluble anodes, and to adjust for zinc, use special installations - zinc generators located outside the galvanizing bath.
Kharlamov V.I.
Addition to Okulov’s article “Behavior of anodes in various galvanizing electrolytes”