From Turkey to Egypt
The history of the discovery of metal has been lost for centuries. Who first discovered the metal, who guessed to “tap” the nugget so that it would turn into a blade or other tool is unknown. Moreover, no one knows who came up with the idea of “welding” the nugget and pouring the liquid metal into the mold.
Copper crystals
The next mystery is who was the first to smelt ore into metal. But it is known that archaeologists found the most ancient finds of copper products and smelting slag in modern Turkey. They are incredibly ancient - they are up to 10,000 years old.
In the Cheops pyramid, archaeologists discovered... a water supply system, and the pipes there were copper. The most amazing thing is that the water supply can still work. I don’t know, however, who needed water there - not the mummies of the pharaoh...
Why copper?
The tribes who lived in Europe in ancient times called copper and any metals “mida”. The word “copper” is also found in ancient Russian texts. Scholars consider the word to be related to the Old German "smid" (blacksmith); or a derivative of Media, a country on the territory of present-day Iran.
In Latin, copper is called cuprum (aes cuprium), from the island of Cyprus. There was a rich metal deposit there. Pliny writes:
“...It is known how long the Roman people used only copper coins. Antiquity itself testifies to the importance of this metal.”
For a long time, the main common coin of the Roman Empire was called ass (aes).
Now “copper” and “cuprum” peacefully share their belonging to the non-ferrous metal.
Story
Copper is one of the first metals that humanity dealt with. This was facilitated by the advantages: high prevalence, availability, relatively low melting point.
People appreciated the benefits of copper eight thousand years ago.
The Copper Age began immediately after the Stone Age:
- Copper artifacts dug up in the territory of modern Turkey are recognized as the oldest. These are beads and decorative overlays.
- Cutting tools and utensils were made from metal.
- The history of the discovery of copper mines in Rus' begins in the Urals two thousand years before the new era. Then there were the Caucasus, Altai, Siberia.
- Industrial processing using bronze began in the 14th century. Cannons and bells were cast from the alloy.
The Tsar Bell and the Tsar Cannon were cast from bronze.
It is assumed that the metal is named after the island of Cyprus. Copper deposits were discovered here back in the 3rd century BC, and the population mastered copper smelting.
M. Vasmer’s “Etymological Dictionary of the Russian Language” links the origin of the Russian term copper with the ancient German root smid – blacksmith, metal.
Metal properties
Copper is in the 11th group of the periodic table of Mendeleev, in the so-called “trinity of expensive metals” - copper, gold, silver. Atomic number 29. The color of the metal is yellowish-pink, close to orange.
In the classification, the element is in the group of transition metals.
The physical properties have been appreciated for a long time, and they are still in demand. This has excellent thermal and electrical conductivity. In these indicators, copper is second only to silver; the presence of impurities (tin, iron, arsenic) worsens the performance.
Pure copper is soft, malleable, and easy to roll. The wire can be brought to a diameter of thousandths of a millimeter.
Density 8.92 g/cm3. Melts at 1083.4 °C, boils at 2567 °C.
| Properties of the atom | |
| Name, symbol, number | Copper/Cuprum (Cu), 29 |
| Atomic mass (molar mass) | 63.546(3)[1] a. e.m. (g/mol) |
| Electronic configuration | [Ar] 3d10 4s1 |
| Atomic radius | 128 pm |
| Chemical properties | |
| Covalent radius | 117 pm |
| Ion radius | (+2e) 73 (+1e) 77 (K=6) rm |
| Electronegativity | 1.90 (Pauling scale) |
| Electrode potential | +0.337 V/ +0.521 V |
| Oxidation states | 3, 2, 1, 0 |
| Ionization energy (first electron) | 745.0 (7.72) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 8.92 g/cm³ |
| Melting temperature | 1356.55 K (1083.4 °C) |
| Boiling temperature | 2567 °C |
| Ud. heat of fusion | 13.01 kJ/mol |
| Ud. heat of vaporization | 304.6 kJ/mol |
| Molar heat capacity | 24.44[2] J/(K mol) |
| Molar volume | 7.1 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 3.615 Å |
| Debye temperature | 315 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 401 W/(m K) |
| CAS number | 7440-50-8 |
We recommend: NON-FERROUS METALS - everything except iron
The crystal structure of the lattice is face-centric, cubic.
The chemical properties of the element are attractive to industry:
- Metallic copper is quite stable and inactive. The spiers of old churches were covered with copper sheets, which regularly protected the roof for many years.
- Exhibits oxidation states of 3, 2, 1, 0.
- The metal does not dissolve in dilute sulfuric and hydrochloric acids, but concentrated nitric acid readily reacts with copper.
- Reacts easily with sulfur and halogens (iodine, fluorine, chlorine).
In nature it consists of the isotopes 63Cu and 65Cu.
Copper connections
Copper sulfate and copper sulfate are most often found in nature. Summer residents and gardeners know this blue powder well. It is used to disinfect plants from insects.
Copper acetate is a fungicide, a component of ceramic paint.
Parisian (Schweinfurt) green, copper acetate arsenide. It is still used to paint the outer parts of sea vessels (so that they do not become overgrown with mollusks and other marine life). Fungicide, insecticide.
Oxides are used in the coloring of glass and enamels.
Nitrates are used for patination of copper products.
Over time, a natural patina forms on them - a greenish oxide-carbonate film. Sometimes patina is increased artificially to age it and give an antique look to the product.
What is
Copper is a pinkish metal with a golden-metallic luster. Element No. 29 of the Mendeleev periodic table. International designation: Cu (Cuprum).
Pure metal is soft, so it is often used with impurities. Plastic: stretches to micron diameters.
In air it becomes covered with a film, acquiring a yellowish-red tint. Thin plates are greenish-blue when exposed to light.
According to the official classification, it is classified as a heavy non-ferrous metal. This group also includes lead, zinc, tin, and nickel.
Looking for copper
There are considerable reserves of metal on earth. These include native copper (its accumulations can reach 400 tons - take ready-made ones).
There is no native copper-containing minerals available to humans:
- copper pyrite (chalcopyrite);
- bornite (copper and iron sulfide); it used to be called motley copper pyrite;
- chalcocite, copper luster (copper sulfide);
- malachite, copper carbonate; Ural malachite of the highest quality has already been used, now malachite is mined in Africa;
- cuprite (red copper ore);
- azurite, copper glaze.
Deposits and production
The origin of copper ores is varied. They are oxide, sulfide, mixed. The majority of sulfide ores on earth (about 90%) contain rich ores. Oxidized minerals are not inferior in metal content.
There are large deposits in Chile (predicted reserves are more than 5 million tons).
Native copper
The richest deposits of native copper are located in the USA (Lake Superior), on the island of Vancuve (Canada), Corocoro (Bolivia).
Native “cosmic” copper has been found in meteorites and on the Moon.
We recommend: COBALT - a generous gift from mountain spirits
In Russia, metal mining is carried out in the Krasnoyarsk Territory (the same Norilsk Nickel).
How to smelt cuprum
Methods for obtaining copper:
- pyrometallurgical (with its help, 90% of the metal is produced);
- hydrometallurgical, the remaining 10%.
Hydrometallurgy consists of a single step: treating (usually low-grade) ore with dilute sulfuric acid and then separating copper metal from the solution. In this case, all associated substances from the ore simply disappear.
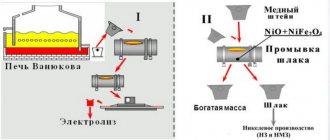
Pyrometallurgy is more complicated, there are several stages:
- Enrichment by flotation and oxidative roasting.
- Melting for matte at temperatures up to 1500 degrees. Here, crude metal is already isolated, as well as accompanying silver, gold, and nickel.
- Fire refining - purification of the resulting metal from impurities to 99.5% purity.
- Electrolytic refining, bringing purity to 99.95%.
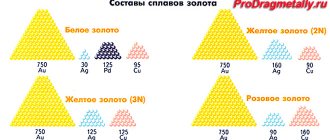
Alloys, alloys...
Copper is part of many alloys:
- cupronickel;
- brass;
- bronze;
- latten (laton);
- German silver (used in jewelry);
- Abyssinian gold;
- French gold;
- northern gold.
Moreover, bronze can be different: tin, bismuth, silicon, aluminum...
The properties of the metal included in the alloy allow metallurgists to “invent” the required characteristics.
Copper is not cheap, so manufacturers prefer copper alloys (where possible).
In some areas, alloys (especially with aluminum) have confidently outperformed pure metal. “Mama” copper cannot compare with the corrosion resistance, strength, and malleability of alloys.
Blue blood
Everyone has heard this expression. Not everyone knows that blue blood actually exists, but not in people. The hemocyanin protein colors the blood of mollusks, centipedes, and arachnids blue. Hemocyanin itself is an analogue of hemoglobin, which makes our blood red.
For biology buffs, the blue blood of horseshoe crabs is used to test medications for contamination.
Without our hero, man would not have been able to survive as a biological species. The metal promotes the absorption of proteins, carbohydrates, and strengthens the immune system. It’s not for nothing that our great-grandmothers cooked jam in copper basins, because the metal has antiseptic properties.
Metal ions have antifungal and antiviral properties, are able to penetrate the cell membrane and destroy the spread of infection.
There is already fabric containing copper threads. You can’t make an evening dress or a suit out of it, but in hospitals it is necessary. The material was developed in Chile.
The World Health Organization believes that a lack of metal in the body can cause illness and worsen blood composition; an excess threatens poisoning, but a lack of metal is more dangerous than an excess. Everything is good in moderation.
Applications of metal: from telegraphs to fireworks
The widespread use of copper began after the invention of the telegraph. Huge amounts of metal were needed for telegraph wires. Since that time, our hero has not left first place in the ranking of electrical metals.
The use of copper is based on its properties. Electrical wiring in old houses; Now expensive metal is being replaced with cheap aluminum. But the devices contain copper wiring. Computers are equipped with copper heat sinks.
We recommend: NICKEL - the “stepson” in the family of silver metals
Plumbing equipment, refrigeration equipment, air conditioners - non-ferrous metal with its remarkable properties is used everywhere.
Ships and boats are proud of their copper pipelines (liquid and gas flow into them).
And in many countries, copper pipes are used for water and gas supply to buildings.
Without copper there will be no brazing solder (that's the "glue" for metals).
Dioskourides wrote: “Solder for gold is prepared from children’s urine and Cypriot copper.”
Japan considers copper gas pipeline pipes to be earthquake-resistant.
Copper is used as an alloy for gold alloys; Pure gold is a metal that is too soft and prone to abrasion.
Orange non-ferrous metal gives blue color to pyrotechnic products.
The service life of copper products reaches 200 years or more.
Popular message topics
- Mausoleum at Halicarnassus
The ancient world boasted an abundance of great cities. Most of them were very beautiful. The city of Halicarnassus was no exception. Many knew it thanks to the abundance of theaters, palaces, and simply beautiful places. Magnificent fountains - Rules of playing basketball
Probably everyone knows that basketball is the most famous and popular sport. Basketball is a sport played by a certain number of people. And each team tries to score the ball in different ways. - Metal processing
Any metal processing is carried out by the metallurgy industry. This is a rather labor-intensive process when a product is obtained from ordinary metal through complex processing. More than half of all available metals that are present in nature are
What we didn't know about copper
One of the advantages of this amazing metal is that tools made from it do not produce sparks when struck. It is wise to use them where there is a risk of explosion.
Swedish scientists have come up with a way to bury radioactive waste. Now huge amounts of money are being spent on this. Or you can simply place radioactive trash in copper capsules with walls 5 centimeters thick. According to calculations, corrosion will destroy them no sooner than in half a million years.
Many people know that the Statue of Liberty (the one with the torch and the crown) is made of copper. Not entirely, of course, non-ferrous metal only on top, steel structures inside. There were rumors that it was made of Ural metal, but... It was officially recognized that it came from Norway.
Here is a case when a seemingly useful property of our hero became a disadvantage. A Norwegian cargo ship sank due to the copper ore it was carrying. Electrochemistry is to blame. Copper from the ore created a galvanic couple with the metal hull of the ship, the evaporation of sea water served as the electrolyte. The resulting current provoked such corrosion that it ate through the lining, and water poured into the holds.
For fashionistas and fashionistas
The secret of the “Gold Vision-3000” jeans is the copper biocorset. Copper threads, “built-in” into fashionable pants, help prevent vascular pathologies and stimulate the functioning of the pelvic organs. And they affect digestion, sexual function, hematopoiesis, reduce the harmful effects of household appliances, and the influence of electromagnetic fields.
Biological significance
It is not so important whether copper is a substance or a body. Something else is significant. What role does copper play in the life of living organisms? It turns out to be quite important. Thus, the ions of the metal in question perform the following functions.
- Participate in the conversion of iron ions into hemoglobin.
- They are active participants in the processes of growth and reproduction.
- They allow the amino acid tyrosine to be absorbed, therefore affecting the appearance of hair and skin color.
If the body does not receive enough of this element in the required quantity, then unpleasant diseases may occur. For example, anemia, baldness, painful thinness, etc.